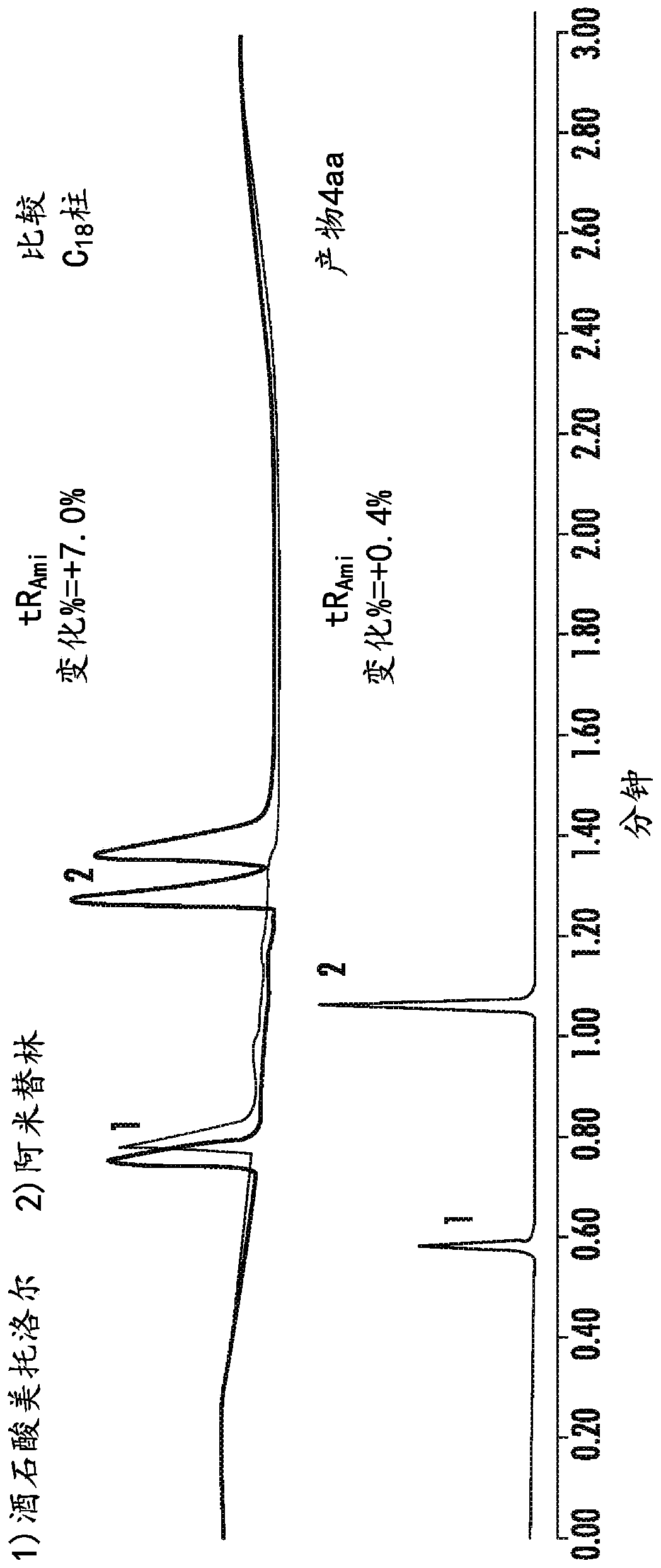
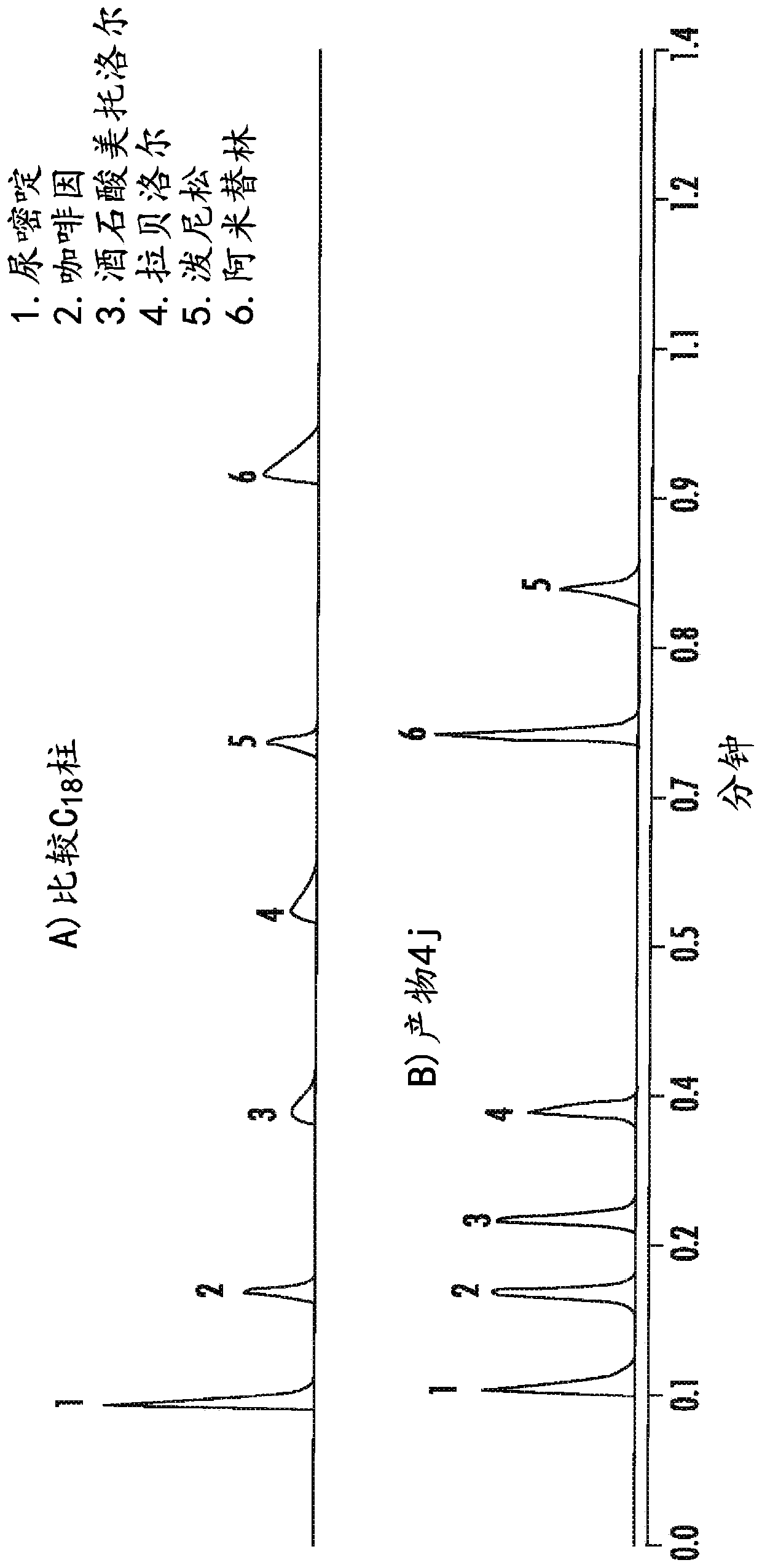
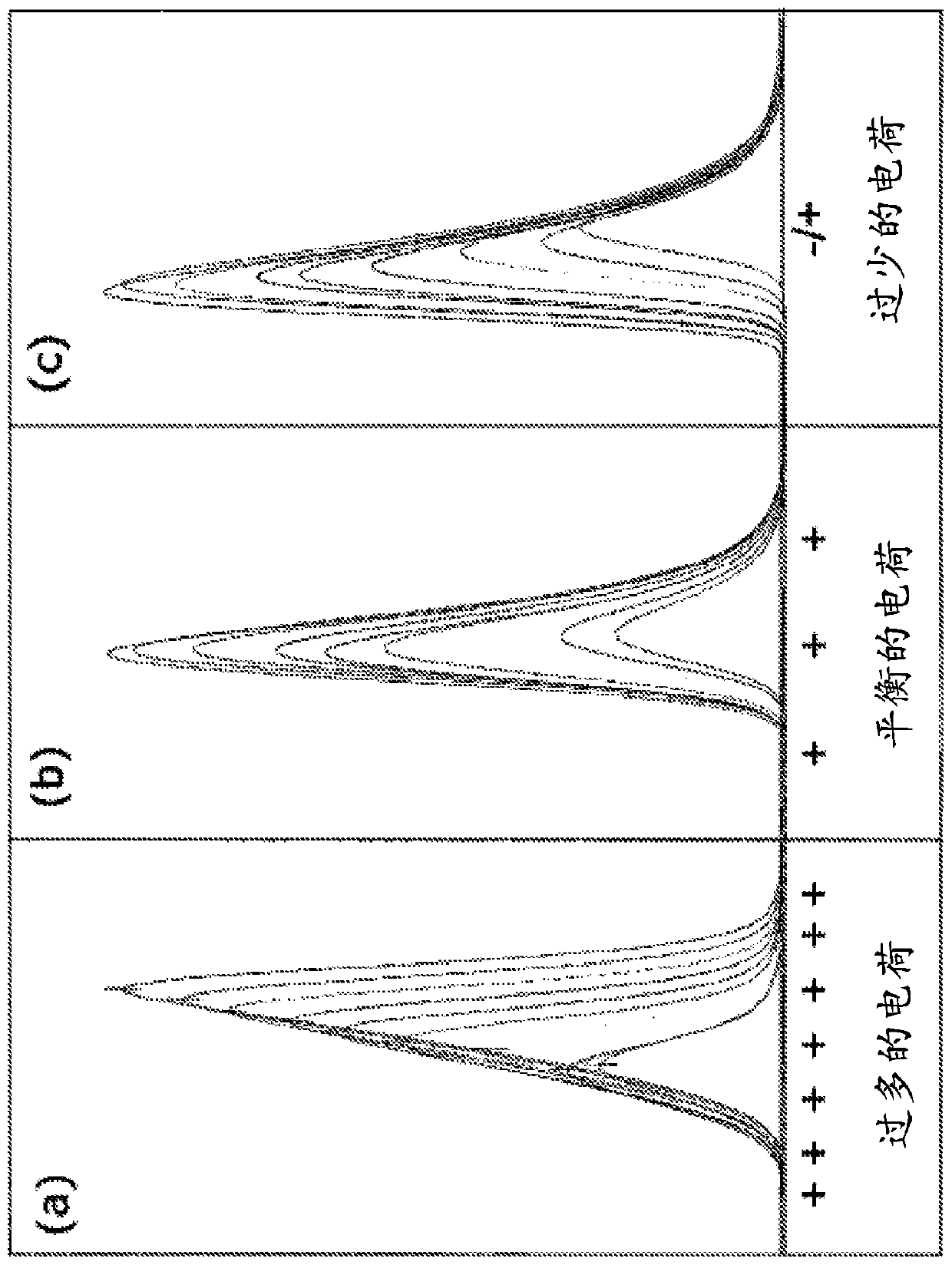
High purity chromatographic materials comprising an ionizable modifier for retention of acidic analytes
A chromatographic material, a high-purity technology for high-purity chromatographic materials that contain ionizable modifiers for retention of acidic analytes, to resolve issues such as sample or diluent detection incompatibilities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0302] will formula (O 1.5 SiCH 2 CH 2 SiO 1.5 ) (SiO 2 ) 4 (Prepared according to the method described in US Patent 6,686,035) BEH porous hybrid particles (15g, Waters Corporation, Milford, MA; 6.5%C; SSA=186m 2 / g; SPV=0.79cm 3 / g; ) was refluxed in toluene (100 mL, Fisher Scientific, Fairlawn, NJ) for 1 hour using a Dean-Stark separator. Reaction 1a used 7.2 g of BEH material. After cooling, add Component A silane additives including aminopropyltriethoxysilane (APTES, Gelest Inc., Morrisville, PA), 2-(2-(trichlorosilyl)ethyl)pyridine (2PE, Gelest Inc., Morrisville, PA), 2-(4-pyridylethyl)triethoxysilane (4PE, Gelest Inc., Morrisville, PA), N-trimethoxysilylpropyl- N,N,N-trimethylammonium chloride (QPTMS, 50% solution in methanol, Gelest Inc., Morrisville, PA) or chloropropyltrimethoxysilane (CPTMS, Gelest Inc., Morrisville, PA). The reaction was heated to reflux for 1 hour. After cooling, imidazole (Aldrich, Milwaukee, WI) and octadecyldimethylchlorosilane (Comp...
Embodiment 2
[0306] The material from Example 1 was modified with trimethylchlorosilane (TMCS, Gelest Inc., Morrisville, PA) using imidazole (Aldrich, Milwaukee, WI) in refluxing toluene (100 mL) for 4 hours. The reaction was then cooled, and the product was filtered and washed sequentially with water, toluene, 1:1 v / v acetone / water, and acetone (all solvents were obtained from J.T. Baker), then dried at 80° C. under reduced pressure for 16 hours. The reaction data are listed in Table 2.
[0307] Table 2
[0308] product precursor Granules (g) TMCS(g) Imidazole (g) %C 2a 1a 7.2 1.49 1.12 13.96 2b 1b 15.9 3.27 2.48 14.22 2c 1c 15.0 3.00 2.25 14.33 2d 1d 15.0 3.11 2.34 13.97 2e 1e 15.6 3.30 2.44 12.93 2f 1f 15.0 3.00 2.25 14.57 2g 1g 15.0 3.11 2.34 14.19 2h 1h 15.0 3.00 2.25 14.19 2i 1i 15.0 3.11 2.34 13.80 2j 1j 15.0 3.00 2.25 13.83 2k 1k 15.0 2.99 2.25 13.12 2l 1l 1...
Embodiment 3
[0310] will formula (O 1.5 SiCH 2 CH 2 SiO 1.5 ) (SiO 2 ) 4 (Prepared according to the method described in US Patent 6,686,035) BEH porous hybrid particles (Waters Corporation, Milford, MA; 6.5% C; SSA=182-185m 2 / g; SPV=0.72-0.76cm 3 / g; ) was refluxed for 1 hour in toluene (5 mL / g, Fisher Scientific, Fairlawn, NJ) using a Dean-Stark separator. After cooling, add Component A silane additives including aminopropyltriethoxysilane (APTES, Gelest Inc., Morrisville, PA), 2-(4-pyridylethyl)triethoxysilane ( 4PE, Gelest Inc., Morrisville, PA) or diethylphosphorylethyltriethoxysilane (DEPS, Gelest Inc., Morrisville, PA) or 2-(4-chlorosulfonylphenyl)ethyltrichloro Silane (SPETCS, 50% solution in toluene, Gelest Inc., Morrisville, PA). The reaction was heated to reflux for 1 hour. After cooling, imidazole (Aldrich, Milwaukee, WI) and octadecyltrichlorosilane (Component B, ODTCS, Aldrich, Milwaukee, WI) were added. The reaction was then heated to reflux for 16 hours. The pro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap