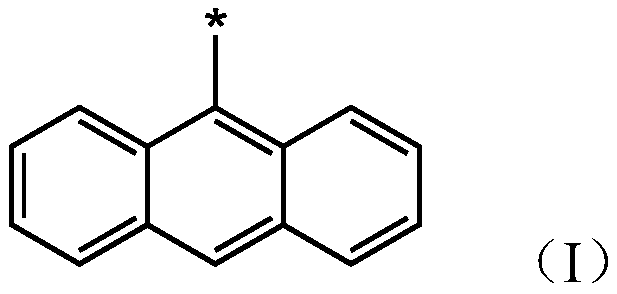
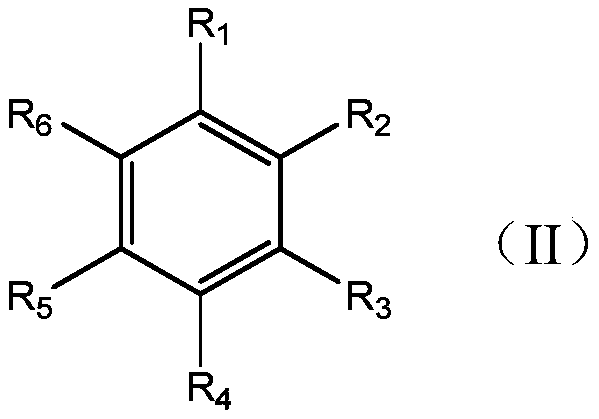
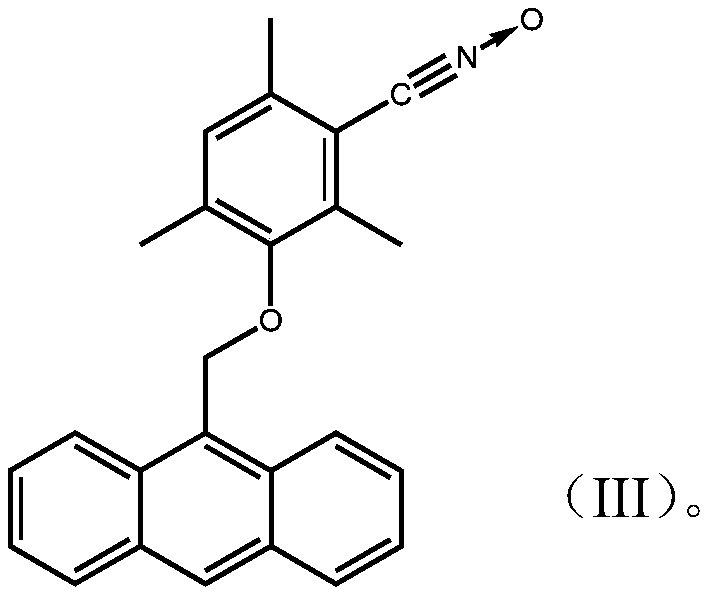
1,3-dipolar compound having conjugated carbon-carbon double bond
A technology of dipolar compounds and dipoles, applied in the field of 1,3-dipolar compounds with conjugated carbon-carbon double bonds, can solve problems such as reactions that have not been described
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0012] I. Detailed description of the invention
[0013] In this specification, all percentages (%) shown are % by weight unless otherwise expressly stated. The abbreviation "phr" denotes parts by weight per hundred parts of elastomer (or the sum of the elastomers if several elastomers are present).
[0014] Furthermore, any numerical interval marked by the expression "between a and b" denotes a numerical range greater than "a" and less than "b" (i.e., excluding the limits a and b), while that marked by the expression "from a to b" Any numerical interval indicates a numerical range extending from "a" up to "b" (ie including the strict limits a and b).
[0015] The expression "composition based on" is understood in this description to mean that the composition comprises a mixture and / or in situ reaction product of the various components used, some of these basic components (e.g. elastomers, fillers or conventionally used Other additives of the rubber composition intended to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com