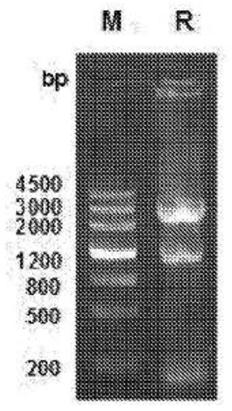
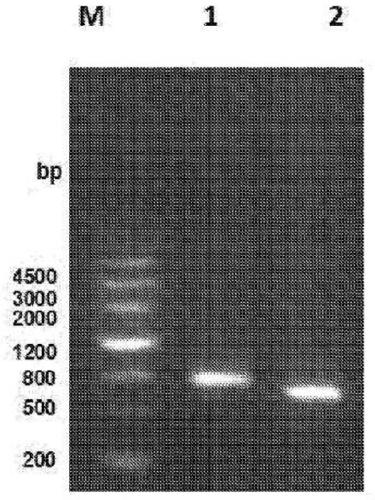
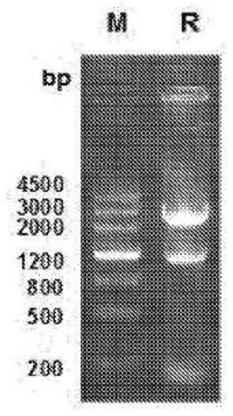
Neutralizing Anti-tl1a monoclonal antibodies
A technology of antibodies and antigens, applied in the direction of antibodies, antibody medical components, anti-inflammatory agents, etc., can solve the problems that hinder the development of new treatments and the limited number of treatments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0165] Generation of immunogens and immunization protocols for hybridoma generation
[0166] The recombinant TL1A protein with the point mutation C66S (counted from the leading methionine in the full-length protein) and lacking the leading 57 amino acids was expressed in E. coli. 重组TL1A序列由SEQ ID NO:33(QLRAQGEASVQFQALKGQEFAPSHQQVYAPLRADGDKPRAHLTVVRQTPTQHFKNQFPALHWEHELGLAFTKNRMNYTNKFLLIPESGDYFIYSQVTFRGMTSECSEIRQAGRPNKPDSITVVITKVTDSYPEPTQLLMGTKSVCEVGSNWFQPIYLGAMFSLQEGDKLMVNVSDISLVDYTKEDKTFFGAFLL)表示。
[0167] HEK293 cell lines expressing TL1A were generated by transduction with a lentiviral construct containing the full-length TL1A protein sequence. This cell line expresses the membrane-bound and secreted forms of the protein (as determined by flow cytometry and ELISA-based methods, respectively). 由HEK293细胞系表达的TL1A的序列由SEQ ID NO:34(MAEDLGLSFGETASVEMLPEHGSCRPKARSSSARWALTCCLVLLPFLAGLTTYLLVSQLRAQGEACVQFQALKGQEFAPSHQQVYAPLRADGDKPRAHLTVVRQTPTQHFKNQFPALHWEHELGLAFTKNRMNYTNKFLLIPESGDYFIYS...
Embodiment 2
[0211] The heavy chain variable region (Table 4) and light chain variable region (Table 5) of the two antibody sequences were compared using BLASTP 2.2.32+ alignment.
[0212] Table 4: BLAST analysis of the heavy chain variable region
[0213]
[0214]
[0215] table 5 : BLAST analysis of light chain variable regions
[0216]
Embodiment 3
[0218] Anti-human TL1A monoclonal antibodies 9E12E5 and 5C3D11 neutralized the activity of human TL1A in vitro. The effect of TL1A antibodies on IFN-γ production was assessed, and both TL1A antibodies showed inhibition of TL1A-induced IFN-γ production as Figure 5 shown. MSD plates were coated with murine TL1A and incubated with various concentrations of 5C3D11 or 9E12E5 to assess the ability of the antibody to recognize murine TL1A as Figure 6 shown. Human TL1A was included as a positive control. 5C3D11 recognizes murine TL1A in a concentration-dependent manner. The binding profiles of the 5C3D11 and 9E12E5 antibodies were assessed using TL1A-transfected HEK293 cell lines and compared to the untransfected parental HEK293 cell lines. Fluorescent staining of anti-TL1A antibodies in TL1A-expressing HEK293 cells was compared to untransfected parental HEK293 cells, as Figure 7 shown. The binding affinity of the anti-TL1A antibody was measured by Biacore, and the dissociati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bmi | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com