Replication-deficient arenavirus particles and tri-segmented arenavirus particles as cancer vaccines
An arenavirus, defective technology, applied in the direction of viruses, viruses/phages, virus antigen components, etc., can solve the problems of chemotherapy side effects and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0630] 8.1 Example 1: The effect between r3LCMV treatment and chemotherapy
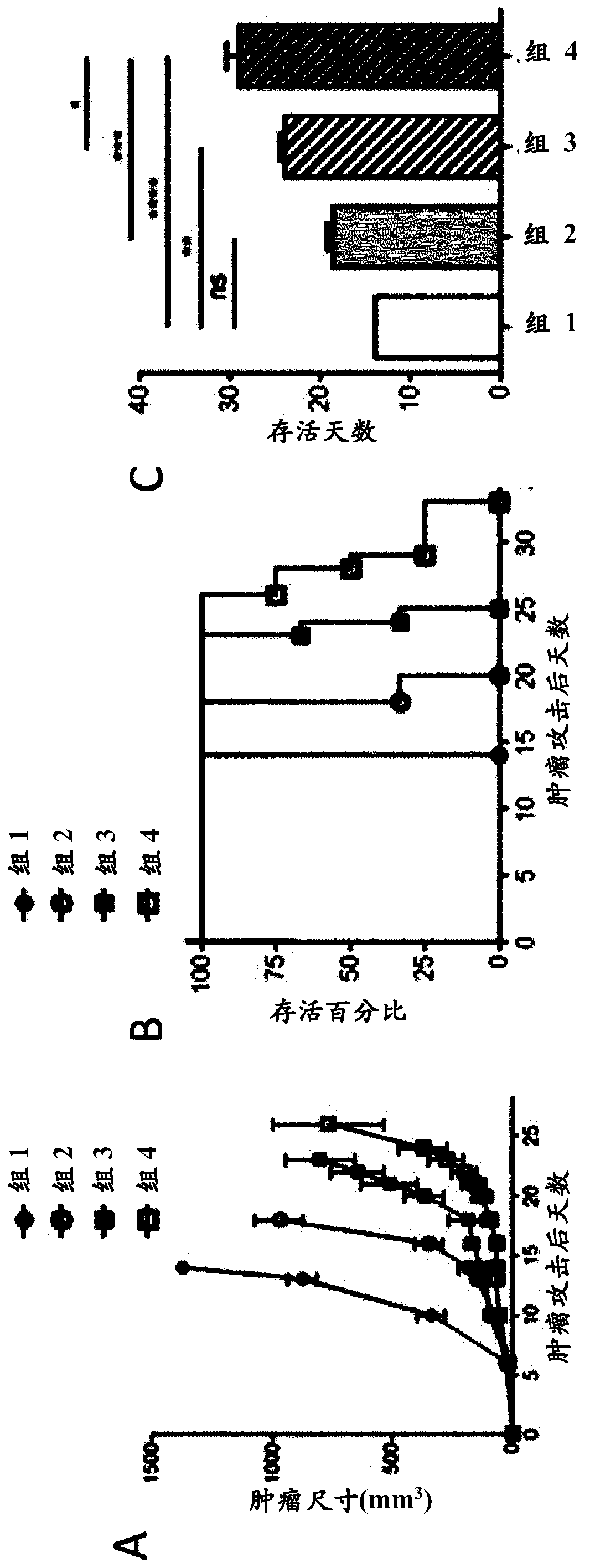
[0631] The potential synergy between r3LCMV treatment and low-dose chemotherapy (cyclophosphamide treatment) was evaluated in the B16F10 mouse melanoma model.
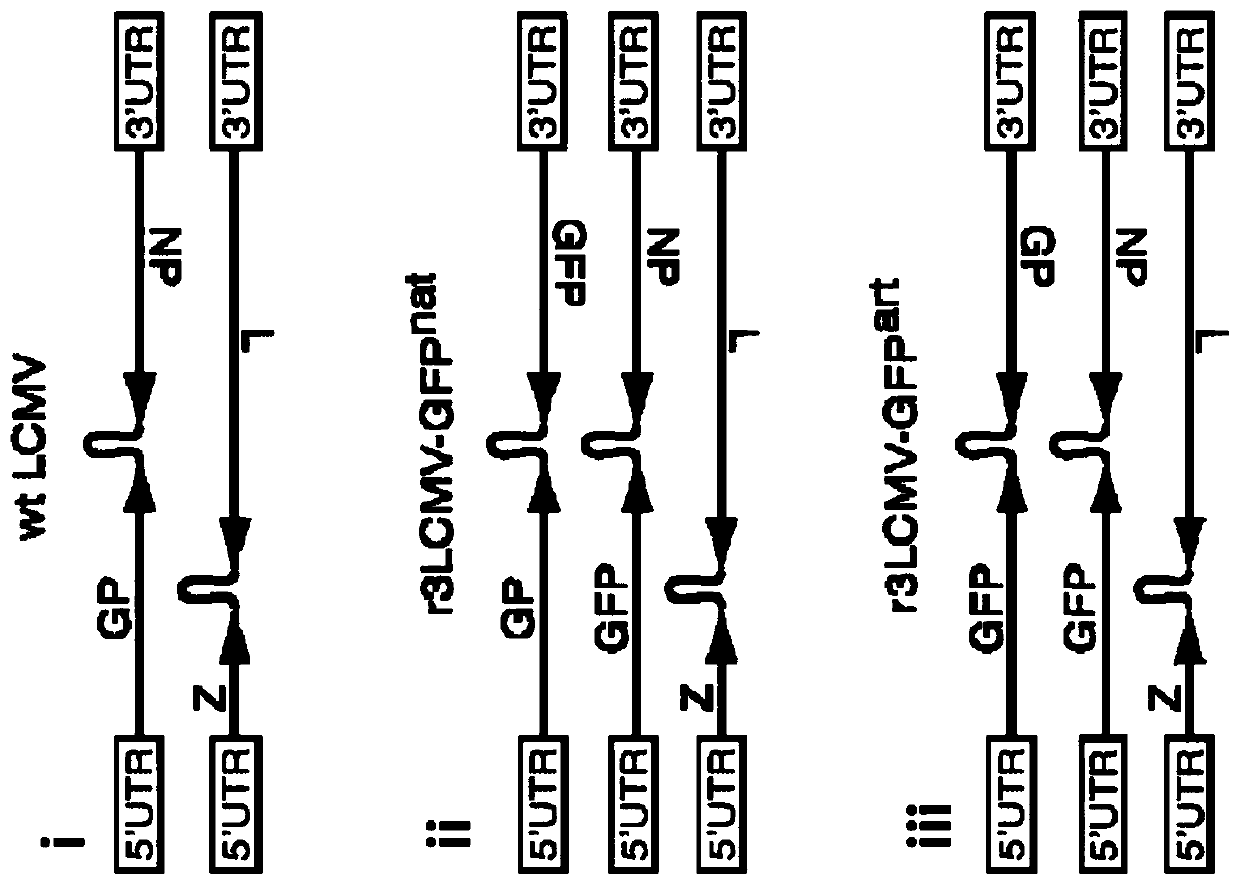
[0632] On day 0, place 1×10 5 B16F10 tumor cells were subcutaneously implanted into C57BL / 6 mice. Subsequently, mice were kept untreated (group 1), treated intraperitoneally with 2 mg cyclophosphamide on day 6 (group 2), and injected intravenously with 2.1 × 10 5 PFU (total) of carrier mix (7 × 10 4 PFU of r3LCMV-GP100, r3LCMV-Trp1 and r3LCMV-Trp2) (group 3), or combined treatment with cyclophosphamide (day 6) and r3LCMV-vehicle mixture (day 7) (group 4). The genome organization of the r3LCMV construct is basically as follows figure 2 For r3LCMV-GFP art As shown, except that the construct has an ORF encoding the antigens of interest, namely GP100, Trp1 and Trp2, instead of the GFP ORF. Tumor growth (Fig. 3A) and survival of animals foll...
Embodiment 2
[0634] 8.2 Example 2: The effect between r3LCMV treatment and chemotherapy in the HCmel3 model
[0635] The potential synergy between r3LCMV treatment and low-dose chemotherapy (cyclophosphamide treatment) was evaluated in the HCmel3 mouse melanoma model. HCmel3 tumor cells are derived from primary Hgf-Cdk4 R24C melanoma.
[0636] On day 0, HCmel3 tumor cells (4×10 5 cells) were subcutaneously implanted into C57BL / 6 mice. Mice in groups 3 and 4 were treated with 2 mg cyclophosphamide (CTX) ip when all tumors were palpable. On day 16, use 7×10 4 Mice in groups 2 and 3 were injected intravenously with RCV FFUr3LCMV-Trp2. Use 1×10 5 Mice in group 4 were immunized intravenously with RCV FFU r3PICV-Trp2.
[0637] The genome organization of the r3LCMV construct is basically as follows figure 2 For r3LCMV-GFP art As shown, except that the construct has an ORF encoding the antigen of interest, Trp2, instead of the GFP ORF. Tumor growth following tumor challenge was monitore...
Embodiment 3
[0639] 8.3 Example 3: HgfxCDK4 R24C / R24C Effects between r3LCMV treatment and chemotherapy in mice
[0640] HgfxCDK4 R24C / R24CThe model is a syngeneic model in which mice develop spontaneous tumors showing some similarities to human melanomas (Landsberg et al., Autochthonous primary and metastatic melanomasin Hgf-Cdk4 R24C mice evade T-cell-mediated immune surveillance. 2010; Bald et al., Immune cell-Poor Melanomas benefit from PD-1 Blockade after targeted typeI IFN activation, 2014).
[0641] in HgfxCDK4 R24C / R24C The potential synergy between r3LCMV treatment and low-dose chemotherapy (cyclophosphamide treatment) was evaluated in a mouse model. Mice remained untreated (group 1) and were treated with 2 mg cyclophosphamide ip when tumors were palpable (approximately day 60) (group 2), and when tumors were palpable (approximately day 60) were treated with The vehicle mixture (r3LCMV-GP100, r3LCMV-Trp1 and r3LCMV-Trp2) was injected intravenously (group 3) or treated with a c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com