Recombinant expression method and application of Brevinin-2GUb polypeptide
An expression method and expression vector technology, applied in the field of recombinant expression of Brevinin-2GUb polypeptide, can solve the problems of high artificial synthesis cost, difficult extraction, complex procedures, etc., achieve significant insulin secretion-stimulating activity, low cost, and simple expression and purification Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
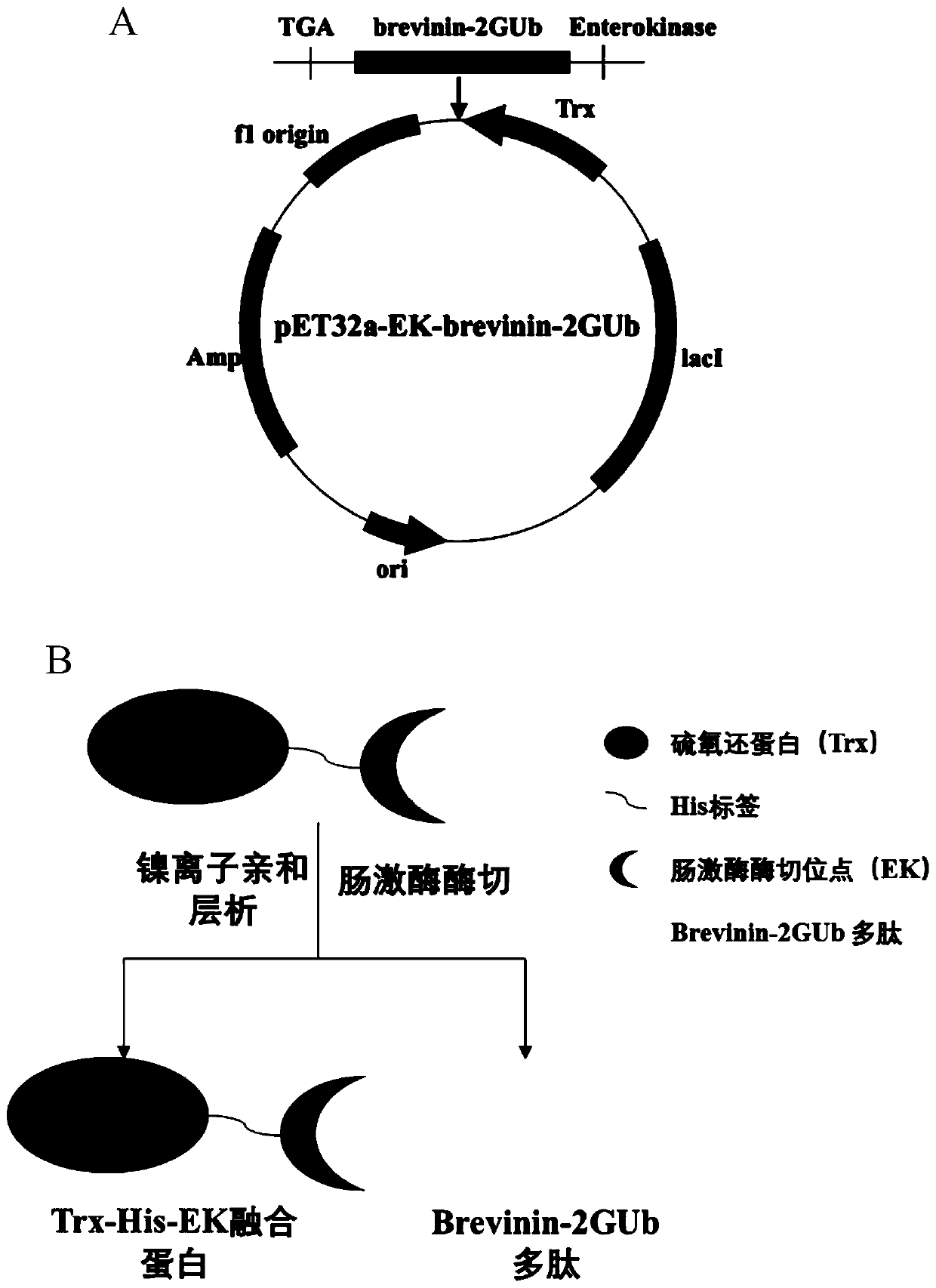
[0039] Example 1: Construction of recombinant expression vector pET32a-Trx-His-EK-Brevinin-2GUb
[0040] (1) According to the gene sequence of Brevinin-2GUb, synthesize its DNA sequence in Gene Synthesis Company (Shanghai Shenggong), design the following primers (Bre-F / Bre-R) to amplify the target gene of Brevinin-2GUb:
[0041]
[0042] (2) Using the synthetic plasmid (pUC57-Brevinin-2GUb) containing the Brevinin-2GUb gene as a template, use the above amplification primers to amplify the Brevinin-2GUb target gene. The specific steps are as follows:
[0043] a. PCR reaction system (50μL):
[0044]
[0045] b. PCR amplification reaction program:
[0046]
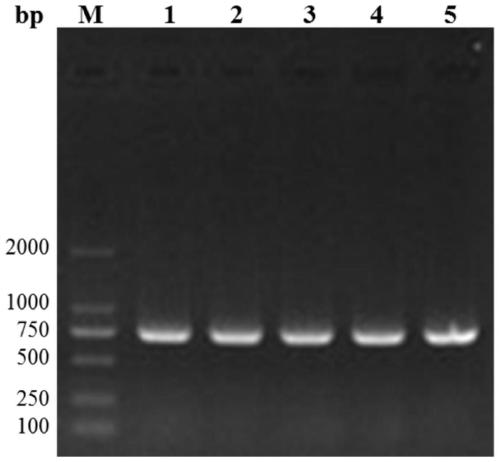
[0047] (3) After the PCR reaction is completed, perform 2% (w / v) agarose gel electrophoresis identification to obtain a gene fragment containing Brevinin-2GUb with a size of about 133bp. Purify and recover the PCR product to obtain Brevinin-2GUb Gene fragments, then prepare a linear amplification reaction system, insert the recovered ...
Embodiment 2
[0061] Example 2: Expression and optimization of Trx-His-EK-Brevinin-2GUb fusion protein
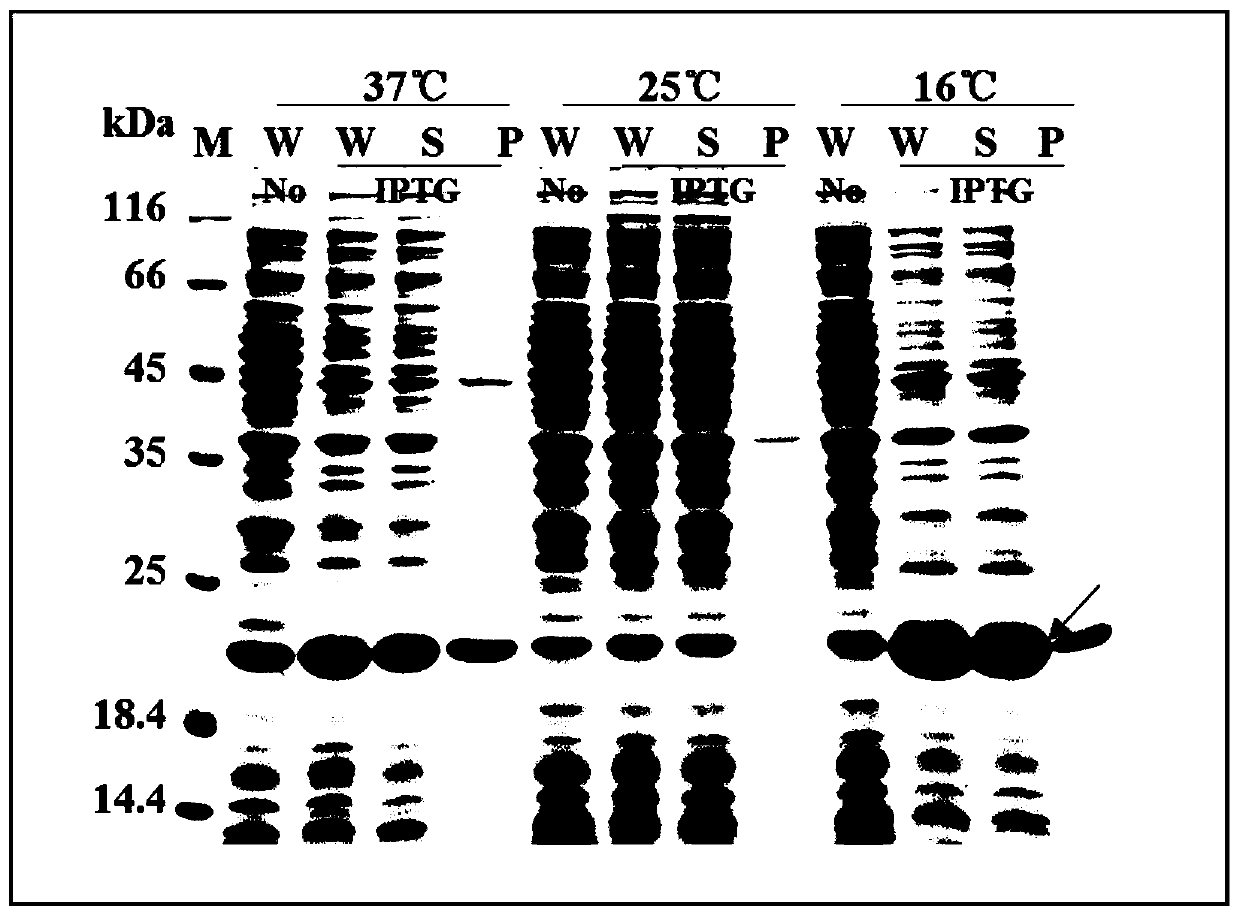
[0062] (1) Transform the recombinant expression vector pET32a-Trx-His-EK-Brevinin-2GUb constructed in Example 1 into E. coli BL21 (DE3) competent cells by chemical transformation, and pick a single clone on the transformed plate for inoculation Into 6 mL of ampicillin-resistant LB liquid medium, cultured overnight at 37°C and 220 rpm as a seed solution. The seed solution was inoculated into 6 test tubes containing 6 mL of fresh ampicillin-resistant LB liquid medium at a ratio of 1:100 (v / v), and incubated at 37°C and 220 rpm. 600 =0.6~0.8, 3 tubes were induced by adding 1mM IPTG, and the other 3 tubes were not added with inducer IPTG as control. One tube was induced and 1 tube was not induced. A total of three groups were induced at 16℃, 25℃, 37℃ for 20h. , 12h, 5h for expression temperature optimization.
[0063] (2) After the induction of expression, the OD of the expressed bacterial solut...
Embodiment 3
[0066] Example 3: Mass expression of Trx-His-EK-Brevinin-2GUb fusion protein and purification by nickel ion affinity chromatography
[0067] (1) The single clone in Example 2 was inoculated into 8 mL of LB liquid medium and cultured overnight at 37°C and 220 rpm as a seed solution. The seed solution was inoculated into 600 mL of fresh ampicillin-resistant LB liquid medium at a ratio of 1:100 (v / v), and incubated at 37°C and 220 rpm. 600 =0.6~0.8, add the inducer IPTG to a final concentration of 1mM, and then place it in a shaker at 16℃ to induce expression for 20h.
[0068] (2) After the expression, the cells were collected by centrifugation at 6000 rpm for 10 minutes, resuspended in purified Buffer A (20mM Tris, 500mM NaCl, 20% glycerol, 20mM imidazole, pH 8.5), crushed by high pressure, centrifuged to collect the supernatant, and then proceeded Purify by nickel ion affinity chromatography, collect the passing fluid sample and the samples before and after purification for SDS-PAGE...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap