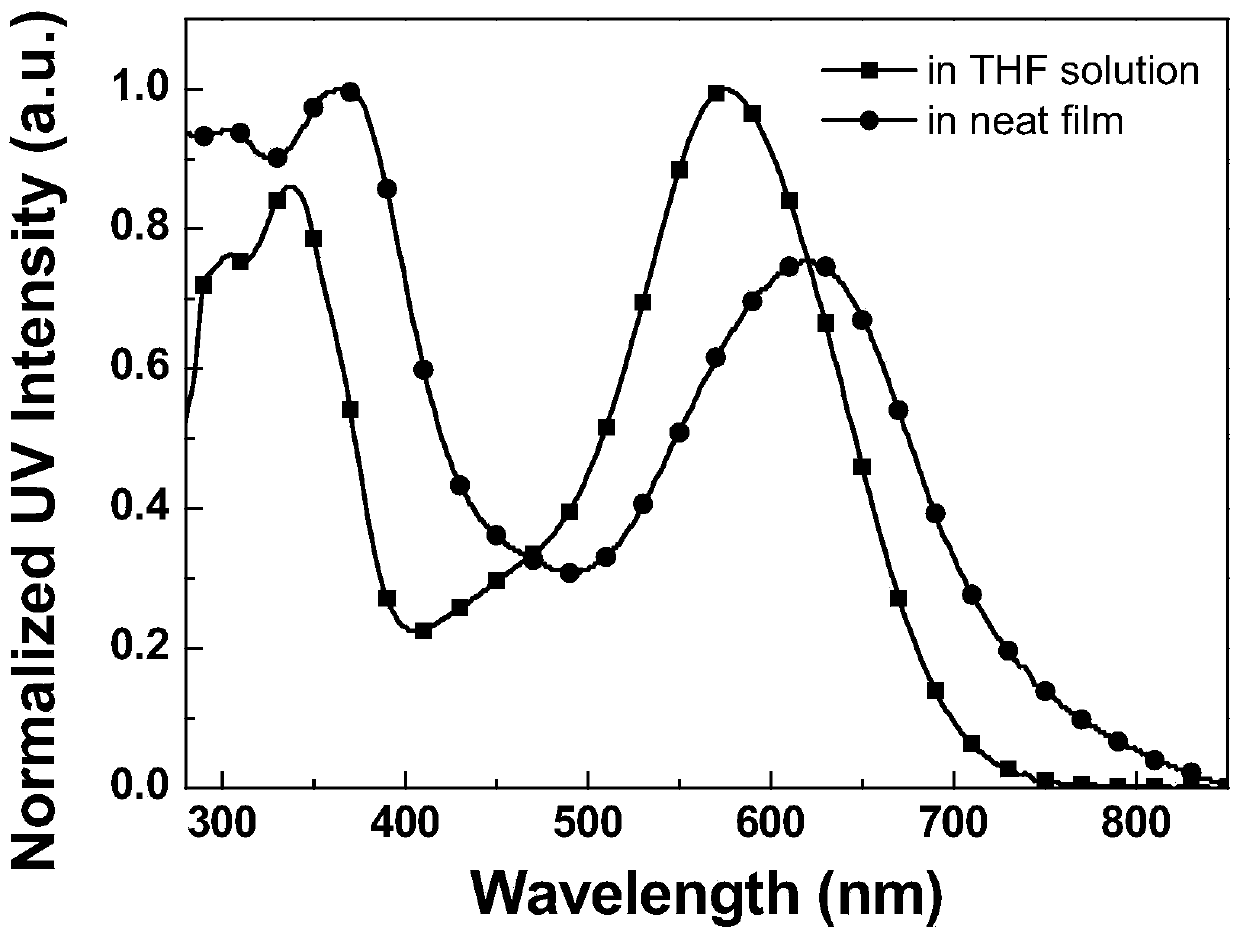
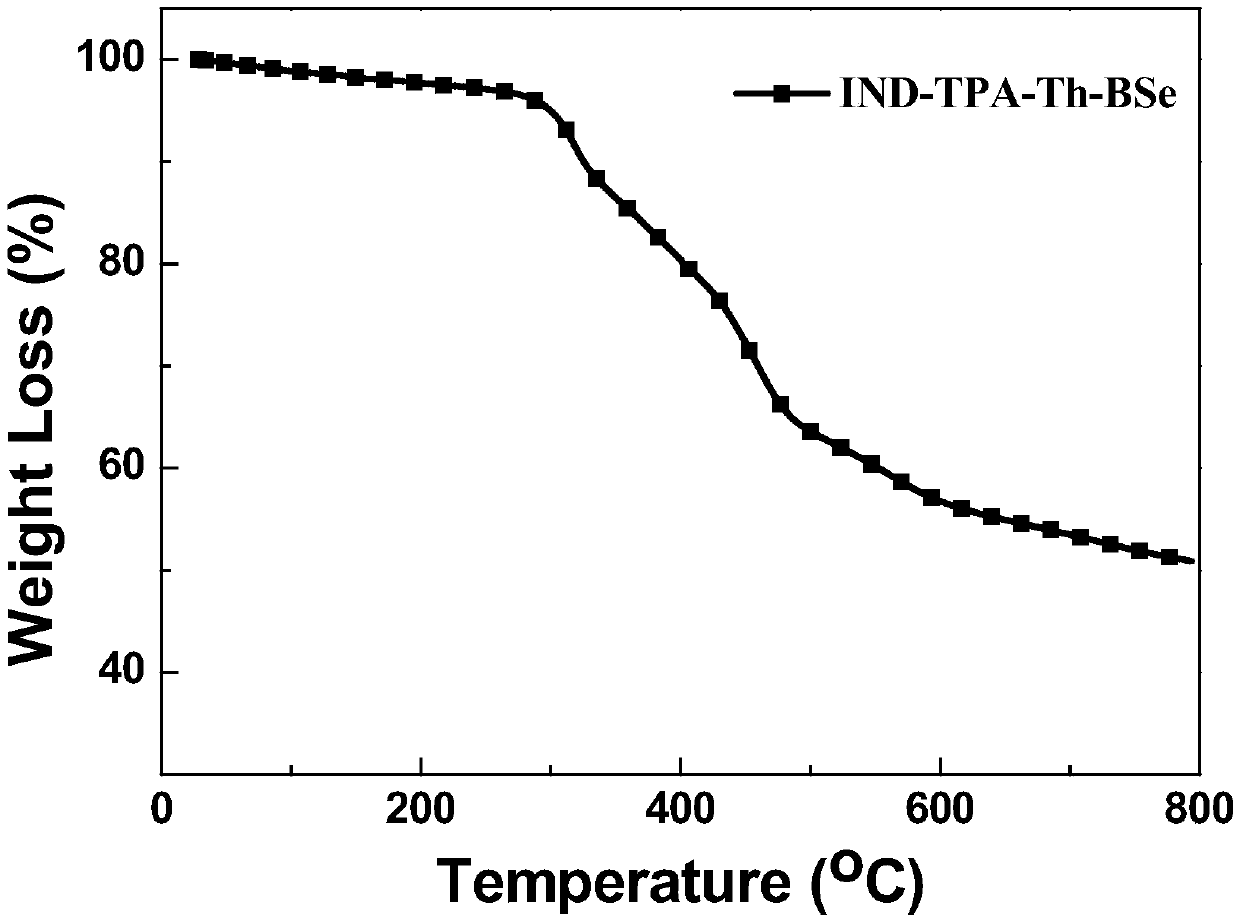
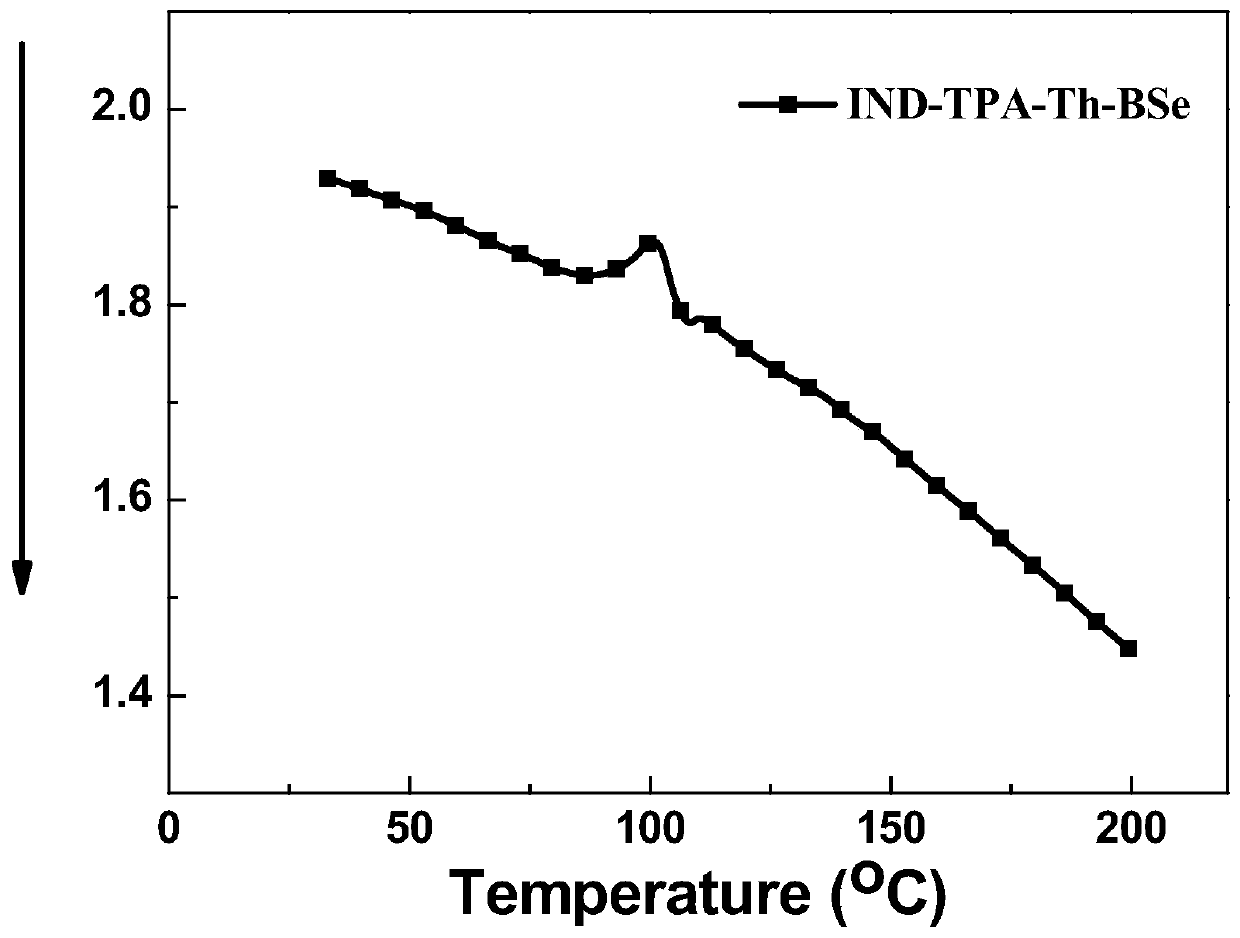
A-D-A-D-A type organic micromolecular solar cell donor materials, and preparation method and application thereof
An organic solvent, volume ratio technology, applied in organic chemistry, electrical solid devices, semiconductor/solid device manufacturing, etc., can solve problems such as device performance impact, analysis, unfavorable structure, etc., to improve device performance, enhance charge transfer, broaden The effect of absorption bands
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Synthesis of 3-(dicyanomethylene)indoketone
[0037]
[0038] Add 1,3-indandione (2.193g, 15mmol) into a 100ml round bottom flask, add sodium acetate (1.599g, 19.5mmol), 30ml of absolute ethanol in turn, add malononitrile (1.981g, 30mmol) Finally, quickly seal the bottle mouth with a drying tube. At this time, the solution in the bottle turns red. After reacting at room temperature for 40 minutes, pour the reaction solution into water, add hydrochloric acid to adjust the pH, add hydrochloric acid dropwise until the solution turns gray, and filter with suction. The filter residue was washed with a large amount of water, and then recrystallized with acetic acid to obtain light red needle-like crystals (2.132g, 10.98mmol, 73.2%). This light red acicular crystal is 3-(dicyano methylene) indoketone, and its nuclear magnetic resonance experiment data is as follows:
[0039] 1 H NMR (400MHz, CDCl 3 )δ8.70–8.63 (m, 1H), 8.00 (d, J=7.5Hz, 1H), 7.95–7.83 (m, 2H), 3.74 (d, J...
Embodiment 2
[0041] Synthesis of 4-{phenyl[4-(4,4,5,5-tetramethyl-1,2,3-dioxaborolan-2-yl)phenyl]amino}benzaldehyde
[0042]
[0043] (1) Synthesis of 4-[-(4-bromophenyl)amino]benzaldehyde
[0044]
[0045] Add 4-bromo-triphenylamine (3.849g, 11.87mmol) into a 100ml three-neck round-bottomed flask, pass nitrogen gas, add anhydrous DMF (12ml, 152mmol) under nitrogen protection to dissolve, cool to 0°C, add trichloroxy Phosphorus (4.33ml, 29mmol) was reacted for 1 hour, heated to 50°C for 12 hours, stopped the reaction, cooled to room temperature, poured the reaction solution into ice water, adjusted the pH to neutral with saturated NaOH aqueous solution, and then raised the temperature to 70°C for reaction 1h, stop the reaction, cool to room temperature, filter with suction, dissolve the filter cake with dichloromethane, then extract with ethyl acetate and water, combine the organic phases, wash the organic phase with water, dry overnight with anhydrous magnesium sulfate, filter the s...
Embodiment 3
[0052] Synthesis of IND-TPA-Th-BSe
[0053]
[0054] (1) 4,4-((([2,1,3]-benzoselenadiazole-4,7-substituent bis(4-dodecylthiophene-5,2-substituent))bis( Synthesis of 4,1-phenylene))bis(aniline)benzaldehyde
[0055]
[0056] 4,7-(3-dodecylthiophen-2-yl)-2,1,3-dibenzoselenodiazol (1.023g, 1.22mmol) and 4-{phenyl[4-(4, 4,5,5-Tetramethyl-1,2,3-dioxaborolan-2-yl)phenyl]amino}benzaldehyde (1.456g, 3.65mmol) was added to a 100ml three-neck round bottom flask, and 10ml absolute ethanol, add 10ml 2M K 2 CO 3 Aqueous solution, add 50ml of toluene to dissolve, pass nitrogen, add tetrakis triphenylphosphine palladium (70mg, 0.061mmol) under the protection of nitrogen, continue to pass nitrogen for 30min, heat up to 80°C under nitrogen protection and reflux for 24h, stop the reaction, cool To room temperature, the solution was poured into water, extracted with dichloromethane, the organic phases were combined, washed with water, dried overnight with anhydrous magnesium sulfate, th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com