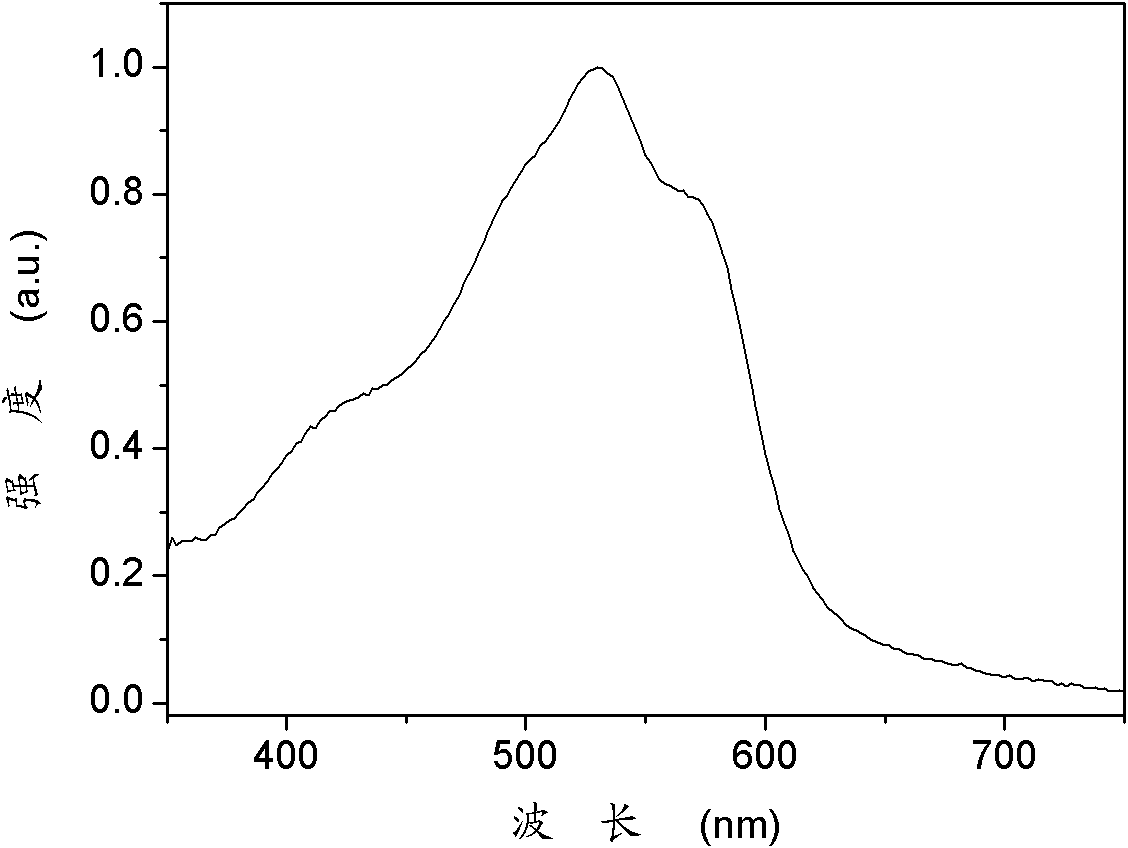
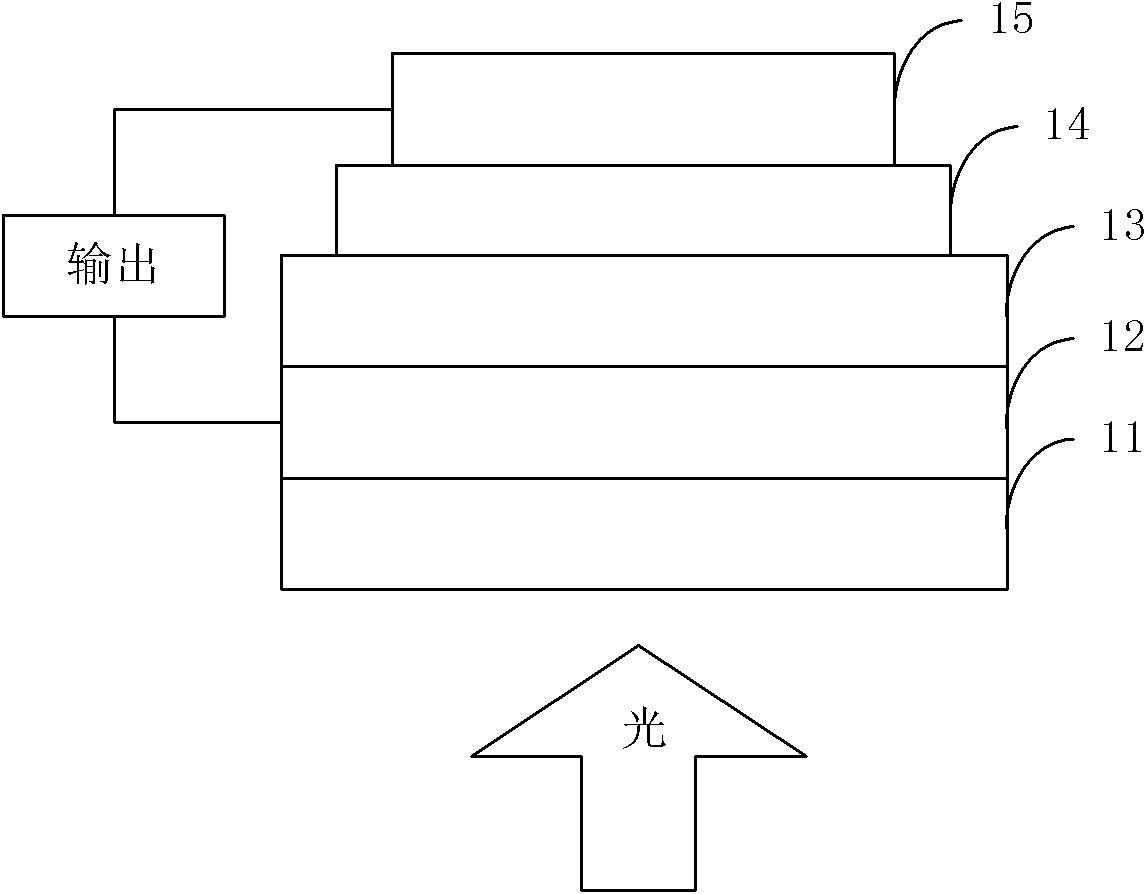
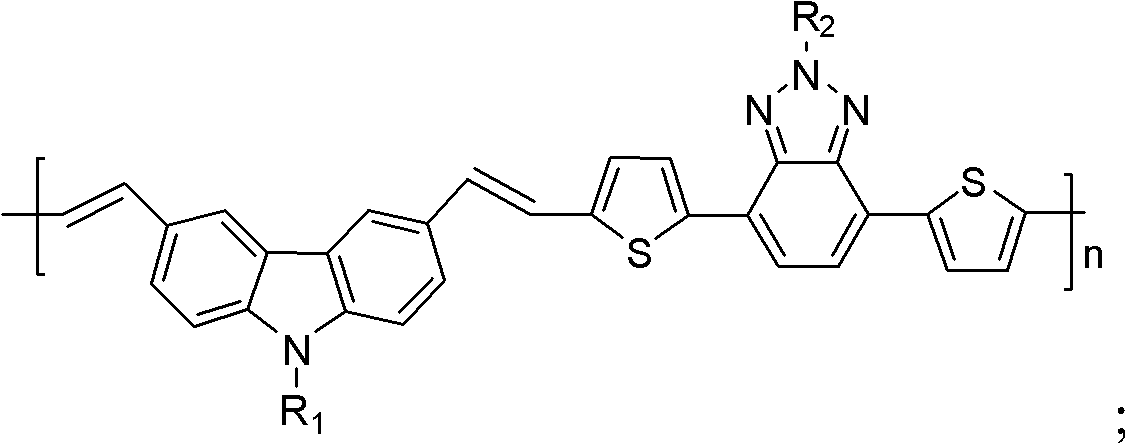
Organic semiconductor material containing benzotriazole group, preparation method thereof and organic solar cell
An organic semiconductor and benzotriazole-based technology, applied in the field of optoelectronic materials, can solve the problems of low utilization rate of sunlight and insufficient absorption of sunlight, and achieve the effects of broadening the absorption band, good photovoltaic performance, and improving utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] A method for preparing a benzotriazole group-containing organic semiconductor material according to an embodiment, comprising the steps of:
[0032] Step S1, respectively provide compound A and compound B of the following structural formula:
[0033]
[0034] Among them, in compound A, R 1 for C 1 ~C 20 the alkyl group; in compound B, R 2 for C 1 ~C 20 the alkyl group;
[0035] Step S2, in an oxygen-free environment (consisting of an inert atmosphere, such as a nitrogen atmosphere or an argon atmosphere), add the compounds A and B to an organic solvent containing a catalyst in a molar ratio of 1:1, and the temperature is 90-120° C. The Heck coupling reaction was carried out for 24 to 72 h to obtain a benzotriazole group-containing organic semiconductor material with the following general structural formula:
[0036]
[0037] In the above formula, n is an integer greater than or equal to 10 and less than or equal to 50; the reaction formula is as follows:
...
Embodiment 1
[0052] The organic semiconductor material containing a benzotriazole group in this Example 1, namely poly{3,6-divinyl-N-octylcarbazole-2-octyl-4,7-dithiophene-1,2, 3-benzotriazole} (wherein R 1 for octyl, R 2 is octyl, n is 25), and its structural formula is as follows:
[0053]
[0054] The preparation steps of above-mentioned polymer are as follows:
[0055] Step 1, the preparation of 2-octyl-4,7-bis(5,5'-dibromothienyl)-1,2,3-benzotriazole, the reaction formula is as follows:
[0056]
[0057] The specific process is as follows: under dark conditions, compound 2-octyl-4,7-dithiophene-1,2,3-benzotriazole (2.37g, 6mmol) was dissolved in chloroform (60mL) and glacial acetic acid (60 mL) mixed solution, moved to an ice bath, then N-bromosuccinimide (2.35 g, 13.2 mmol) was added in batches, and reacted at room temperature for 12 h to obtain a reaction mixture.
[0058] The reaction mixture was extracted with dichloromethane three times and washed with water. The organi...
Embodiment 2
[0067] The organic semiconductor material containing a benzotriazole group in Example 2 is poly{3,6-divinyl-N-dodecylcarbazole-2-hexyl-4,7-dithiophene-1,2 , 3-benzotriazole} (wherein, R 1 is dodecyl, R 2 is hexyl, n is 32), and its structural formula is as follows:
[0068]
[0069] The preparation steps of above-mentioned polymer are as follows:
[0070] Step 1, the difference with step 1 in embodiment 1 is: hexyl replaces octyl;
[0071] Step 2, the preparation of poly{3,6-divinyl-N-dodecylcarbazole-2-hexyl-4,7-dithiophene-1,2,3-benzotriazole}, the reaction formula is as follows shown:
[0072]
[0073] Under nitrogen, 3,6-divinyl-N-dodecylcarbazole (116 mg, 0.3 mmol), 2-n-hexyl-4,7-dibromo-benzotriazole (157.5 mg, 0.3 mmol) ) and 10mL of toluene were respectively added to the two-necked bottle of 50mL, fully vented with nitrogen for about 20min, then added 15mg of bistriphenylphosphonium palladium dichloride, fully vented with nitrogen for about 10min, at 120°C ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com