A scar repair material and preparation method thereof
A technology for repairing materials and scars. It is applied in the direction of medical formulas, medical preparations with non-active ingredients, and medical preparations containing active ingredients. It can solve problems such as poor effect, short action time, and single function, and achieve soft texture. , promote healing, high safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
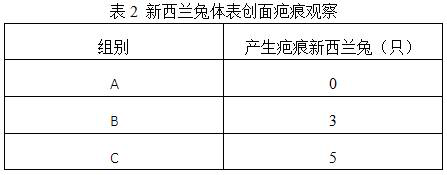
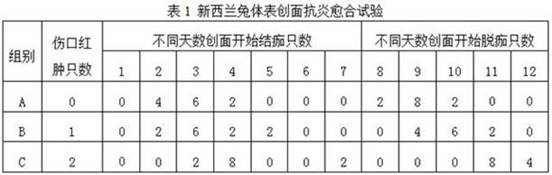
[0037] Three groups of scar repair materials A, B, and C were prepared, and the differences in the efficacy of these three groups of materials for scar prevention and repair in New Zealand rabbits were compared.
[0038] 1. Prepare microcapsules, using water-soluble chitosan and sodium hyaluronate as capsule materials, ectoine and allantoin as core materials, and the viscosity of water-soluble chitosan used is 600-800 mPa·s, The molecular weight of sodium hyaluronate used is 1500 kDa, the mass ratio of water-soluble chitosan and sodium hyaluronate is 1:1, the mass ratio of ectoine and allantoin is 100:1, the capsule material and core material The mass ratio is 10:1. The specific steps are: add chitosan to 0.6wt% acetic acid solution, mix well, add anhydrous calcium chloride, stir and dissolve in a water bath at 45°C until it is completely emulsion-like, and it is system 1; add sodium hyaluronate Stir in purified water until completely dissolved, then add a mixed solution cont...
Embodiment 2
[0047] Scar repair materials were prepared, and the commercially available silicone scar repair materials were selected as the positive control group, and the differences in their effects on new scar repair were compared.
[0048] Preparation of test group materials:
[0049] 1. Prepare microcapsules, using chitosan hydrochloride and sodium hyaluronate as capsule materials, ectoine and allantoin as core materials, and the viscosity of chitosan hydrochloride used is 200-400 mPa. s, the molecular weight of sodium hyaluronate used is 1000 kDa, the mass ratio of chitosan hydrochloride to sodium hyaluronate is 3:1, the mass ratio of ectoine and allantoin is 1:100, the capsule material The mass ratio to the core material is 3:1. The specific steps are: add chitosan hydrochloride into 1wt% acetic acid solution, mix evenly, add anhydrous calcium chloride, stir and dissolve in a water bath at 50°C until it is completely emulsion-like, which is system 1; add hyaluronic acid Add sodium...
Embodiment 3
[0061] Prepare scar repair materials and examine their mechanical strength.
[0062] 1. Prepare microcapsules, using chitosan hydrochloride and sodium hyaluronate as capsule materials, ectoine and allantoin as core materials, and the viscosity of chitosan hydrochloride used is 400~600 mPa· s, the molecular weight of sodium hyaluronate used is 2000 kDa, the mass ratio of chitosan hydrochloride to sodium hyaluronate is 3:1, the mass ratio of ectoine and allantoin is 1:100, the capsule material The mass ratio to the core material is 3:1. The specific steps are: add chitosan hydrochloride into 1wt% acetic acid solution, mix evenly, add anhydrous calcium chloride, stir and dissolve in a water bath at 50°C until it is completely emulsion-like, which is system 1; add hyaluronic acid Sodium was added into purified water and stirred until it was completely dissolved, then a mixed solution of allantoin, ectoine and DMSO was added and mixed evenly. The mass of DMSO was 40% of the mass o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com