Preparation method of Azoxystrobin and intermediate thereof
一种化合物、当量的技术,应用在嘧菌酯及其中间体的制备领域,能够解决反应总收率低、原材料成本高等问题,达到反应总收率高、降低原材料成本的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
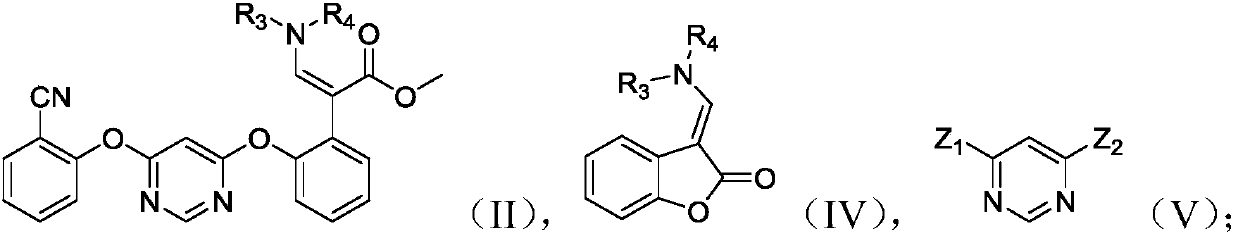
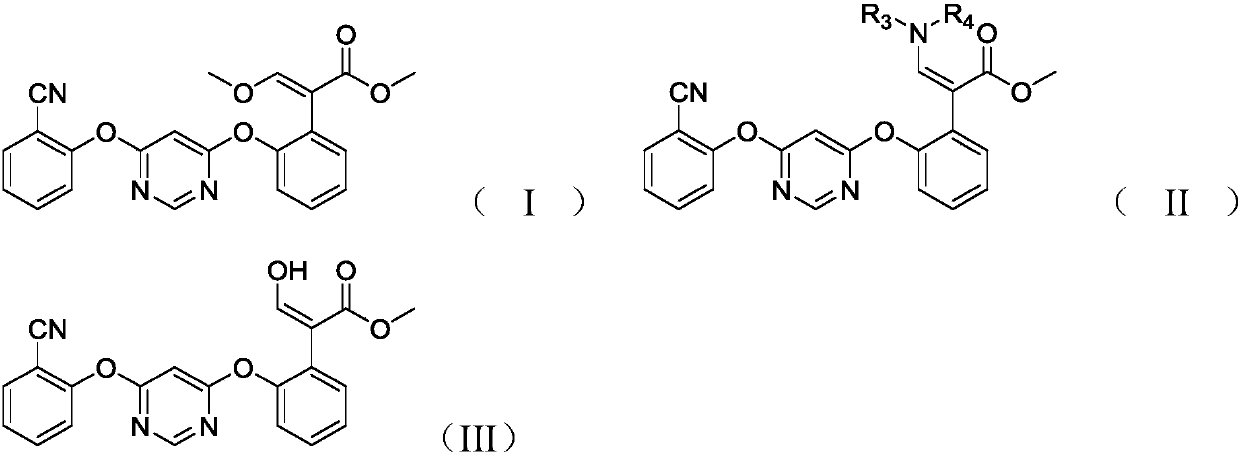
[0037] The present invention also provides a preparation method of the compound shown in formula (I), the method comprising the following steps:
[0038] (1) Under acidic conditions, the compound shown in the formula (II) prepared above is hydrolyzed in an organic solvent to prepare the compound shown in the formula (III);
[0039] (2) reacting the compound shown in the formula (III) with a base and a methylating reagent to prepare the compound shown in the formula (I);
[0040]
[0041] In the formula, R 3 for hydrogen or C 1 -C 4 Alkyl, R 4 for C 1 -C 4 alkyl.
[0042] In the present invention, the cost of raw materials for preparing azoxystrobin is low, and the total reaction yield of azoxystrobin is greatly improved by using the intermediate of azoxystrobin, that is, the compound represented by formula (II) as a raw material.
[0043] In the present invention, based on the amount of the compound represented by formula (II) as 1.00 molar equivalents, the amount of...
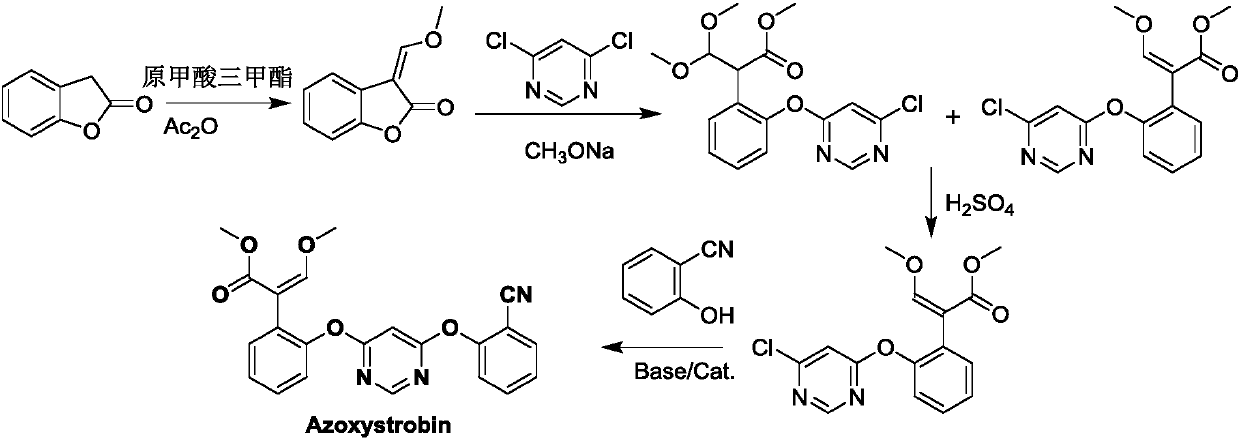
Embodiment 1
[0058] Preparation of 2-(2-(6-chloropyrimidine-4-oxyl group) phenyl)-3-dimethylaminoacrylate methyl ester (ie Z 1 is chlorine, R 3 , R 4 is the general formula (II) compound of methyl)
[0059] In the four-neck flask equipped with mechanical stirring, thermometer, and condenser tube, add 19.5g (0.1mol, 99%) dimethylaminomethenyl benzofuranone (that is, in formula IV, R 3 , R 4 methyl compound) and 150mL toluene, followed by adding 5.5g (0.1mol, 99%) solid sodium methoxide, stirring the reaction at room temperature for 3h, cooling to -15°C, then adding 0.1g triethylenediamine, and simultaneously adding 4, 6-dichloropyrimidine was stirred for 5 hours and then raised to room temperature, washed with water and separated, and the organic phase was concentrated to obtain a solid that was 2-(2-(6-chloropyrimidine-4-oxyl)phenyl)-3-dimethylaminoacrylic acid The methyl ester was directly carried out to the next step reaction without separation. It was measured that the yield of met...
Embodiment 2
[0061] The preparation of 2-(2-(6-(2-cyanophenoxy group) pyrimidine-4-oxyl group) phenyl)-3-dimethylaminoacrylate (i.e. in the formula (II), Z 1 is 2-cyanophenyl, R 3 , R 4 for methyl compounds)
[0062] In a four-neck flask equipped with mechanical stirring, a thermometer, and a condenser tube, add 34 g (0.1mol, 98%) of the product of Example 1 2-(2-(6-chloropyrimidine-4-oxyl)phenyl)- 3-Methylaminoacrylate, 12.0g salicylonitrile and 100mL N,N-dimethylformamide, add 15.5g (0.1mol, 98%) potassium carbonate, add 0.1g triethylenediamine, heat up to 80 ℃ and stirred for 3 hours, then distilled off the solvent N,N-dimethylformamide under reduced pressure, then added toluene to dissolve, and washed with water to separate layers, and the solid obtained by concentrating the organic phase was 2-(2-(6-(2-cyano (phenoxy)pyrimidin-4-oxy)phenyl)-3-dimethylaminoacrylate methyl ester. It was measured that the yield of methyl 2-(2-(6-(2-cyanophenoxy)pyrimidine-4-oxyl)phenyl)-3-dimethylami...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com