Oil based formulations for sublingual and buccal delivery
A delivery system and agonist technology, applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, pill delivery, etc., can solve problems such as discomfort, inconvenience, pain, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
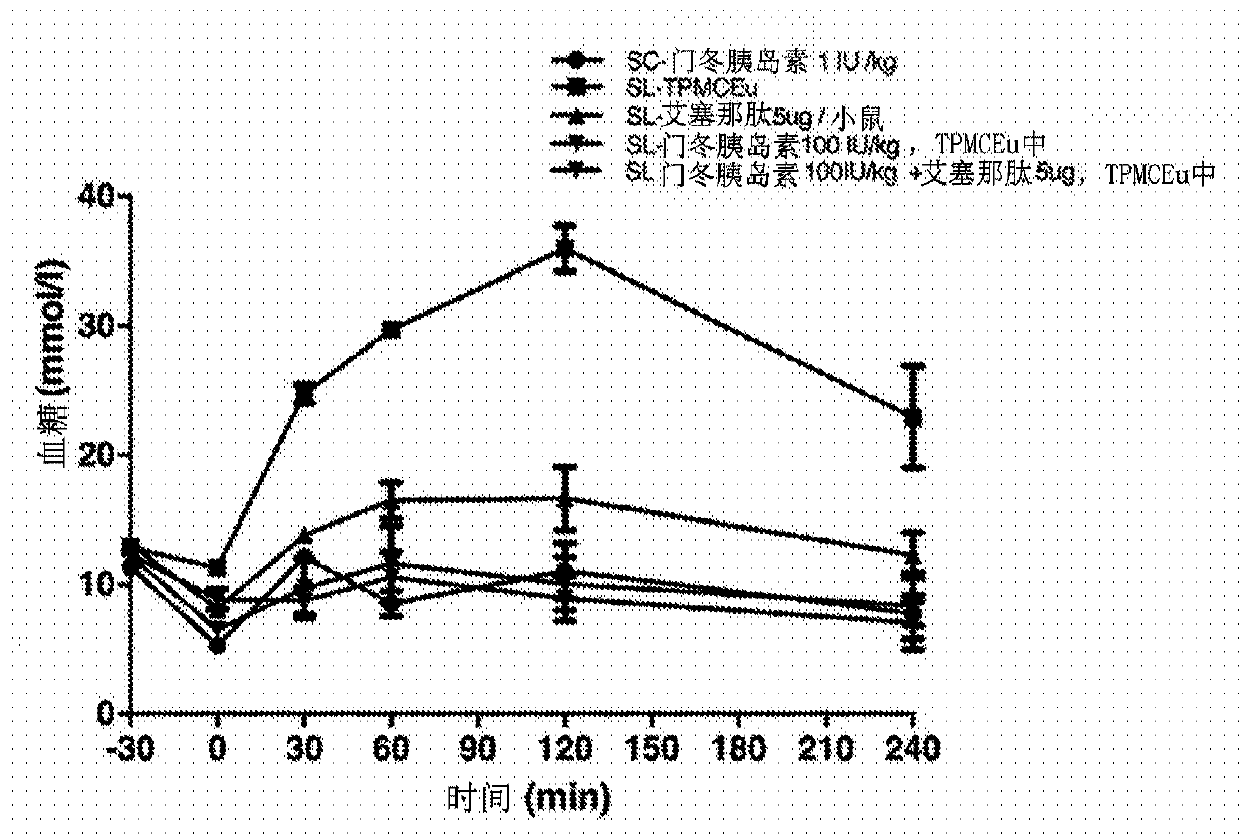
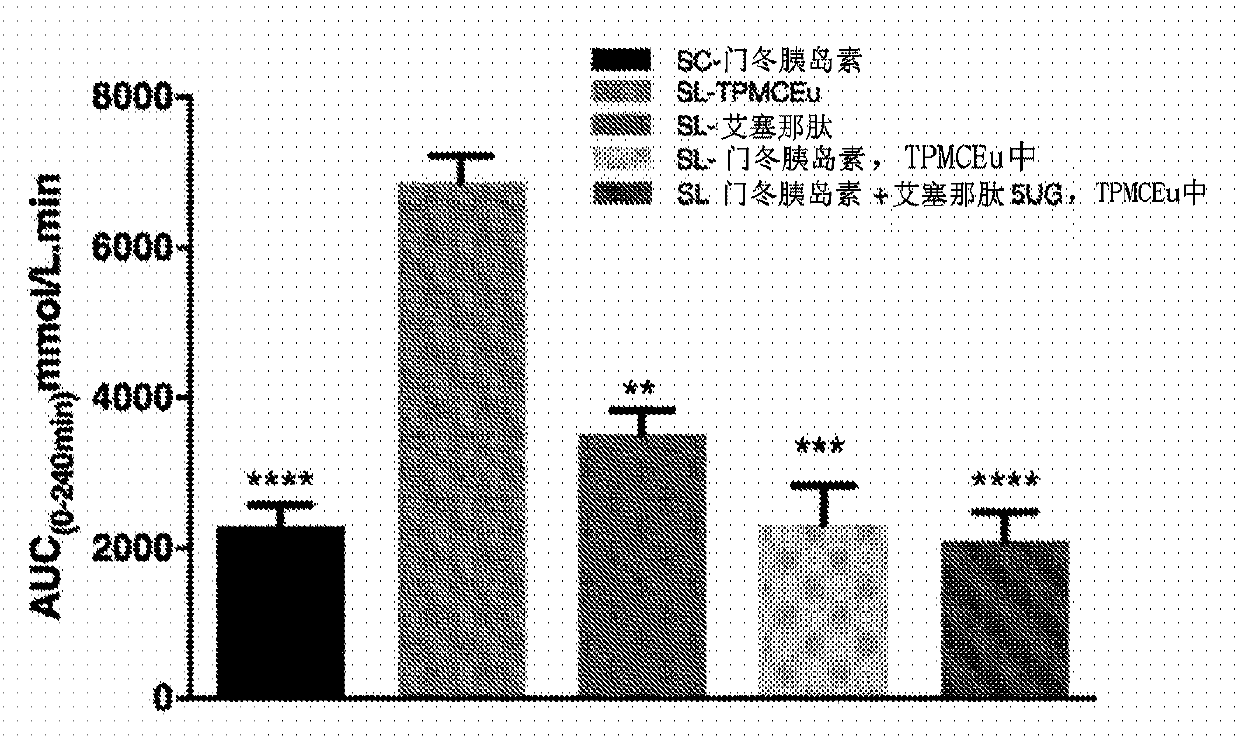
[0098] Example 1 - Sublingual Insulin Aspart Study
[0099] Dissolve insulin aspart (a rapid-acting insulin analog), exenatide, or a mixture of insulin aspart and exenatide in a solution containing 20% vitamin E oil, 20% polyethylene glycol 200, 20% chlorobutanol, and 40% peppermint alcohol / eucalyptol diluent / flavored oil (TPMCEu) and swirl the mixture until homogeneous.
[0100] Male diabetic mice (C57 / BL6 strain) were from Shanghai SLAC Laboratory Animal Co. Ltd. (Shanghai, China). For the experiments described below, mice of approximately 60 g were used. 5 μL of the resulting oil mixture containing insulin aspart was placed under the tongue of an anesthetized diabetic mouse. Blood glucose was measured at -30 minutes, 0 minutes, 30 minutes, 60 minutes, 120 minutes and 240 minutes.
[0101] Five groups of five mice each were treated. The treatment groups were administered as follows: 1) 5 μg sublingually administered exenatide (SL-exenatide); 2) 100 U / kg sublingually ad...
Embodiment 2
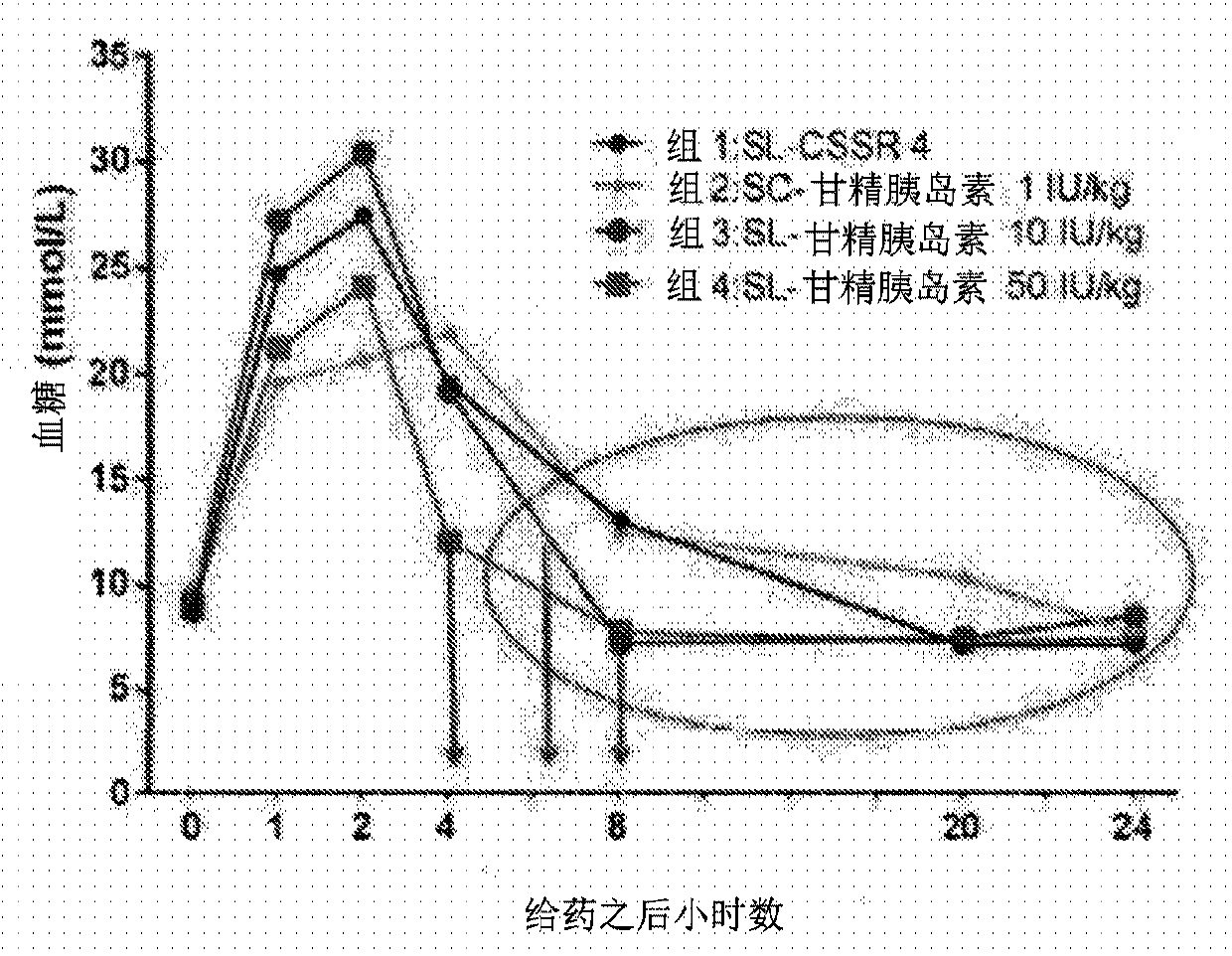
[0104] Example 2 - Sublingual insulin glargine study
[0105] Insulin glargine (a long-acting insulin analog) was dissolved in an oil containing 20% vitamin E oil, 20% polyethylene glycol 200, 20% chlorobutanol, and 40% menthol / eucalyptol diluent / flavor oil ( TPMCEu), the mixture is swirled until homogeneous and absorbed into the solid dosage formulation.
[0106] Male diabetic mice (C57 / BL6 strain) were from Shanghai SLAC Experimental Animal Co., Ltd. (Shanghai, China). One solid dose formulation was placed under the tongue of an anesthetized diabetic mouse. Blood glucose was measured at 0 hours, 1 hour, 2 hours, 4 hours, 8 hours, 20 hours and 24 hours.
[0107] Four groups of five mice each were treated. Treatment groups were administered as follows: 1) Sublingual solid dose formulation placebo (Group 1: SL-CSSR 4; where "CSSR-4" means a solid formulation comprising vitamin E, PEG 200 and flavor oil). 2) 1 IU / kg subcutaneous insulin glargine (group 2: SC-insulin glargi...
Embodiment 3
[0110] Example 3 - "InsulinPlus" Sublingual Insulin Aspart / Insulin Glargine / Exenatide Study 1
[0111] Dissolve insulin aspart, exenatide, and / or insulin glargine in diluent / flavored oil containing 20% vitamin E oil, 20% polyethylene glycol 200, 20% chlorobutanol, and 40% menthol / eucalyptol oil ("CSSR6b") and swirl the mixture until homogeneous.
[0112] Male diabetic mice (C57 / BL6 strain) were from Shanghai SLAC Experimental Animal Co., Ltd. (Shanghai, China). For the experiments described below, mice of approximately 50 g were used. 5 μL of the resulting oil mixture containing the active agents was placed under the tongue of an anesthetized diabetic mouse. Blood glucose was measured at -30 minutes, 0 minutes, 30 minutes, 60 minutes, 120 minutes, 240 minutes, 480 minutes and 720 minutes. Mice were allowed to eat 4 hours later.
[0113] Four groups of five mice each were treated. The treatment groups were administered as follows: 1) Sublingual oil formulation placebo (G...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com