Treatment of hidradenitis suppurativa
A technology for hidradenitis suppurativa and subjects, applied in the fields of medicine, dermatology and immunology, can solve problems affecting the quality of life of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0046] Example 1 - MABpl (True Human Targeting Interleukin-1α TM A double-blind, randomized, placebo-controlled clinical trial of the safety and efficacy of antibody) in HS patients.
[0047] Patients with HS were screened from those currently undergoing follow-up. Inclusion criteria were: written informed consent provided by the patient; age 18 or older; diagnosis of HS; HS with Hurley stage II or III disease or rapidly progressive HS with Hurley stage I; More inflamed nodules; at least one of the following: a) failure of previous treatment with any anti-TNFα regimen; b) recurrence after previous treatment with any anti-TNFα regimen; or c) reluctance to receive subcutaneous adalimumab treatment.
[0048] Exclusion criteria were: history of systemic lupus erythematosus, rheumatoid arthritis, or seronegative inflammatory arthritis; use of any biologic or investigational agent within the past 4 weeks (or 5 half-lives, whichever is longer) Treatment; severe hypersensitivity or ...
example 2
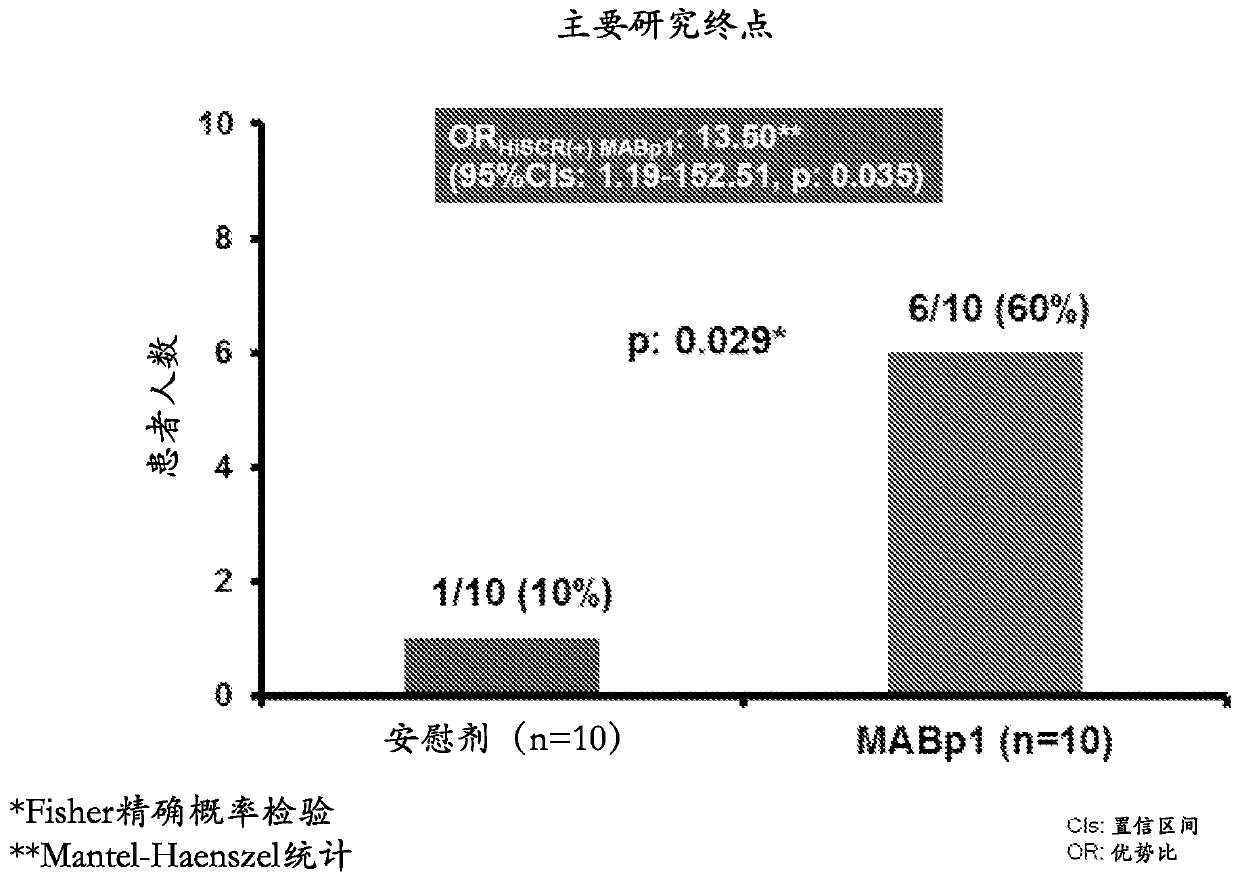
[0076] Key results from an investigator-sponsored randomized phase 2 study evaluating MABpl as a treatment for hidradenitis suppurativa (HS) showed that the study met its primary endpoint, demonstrating significant improvement in HS patients compared to controls after 12 weeks of treatment (Response rates 60% vs. 10%, respectively (p=0.035)).
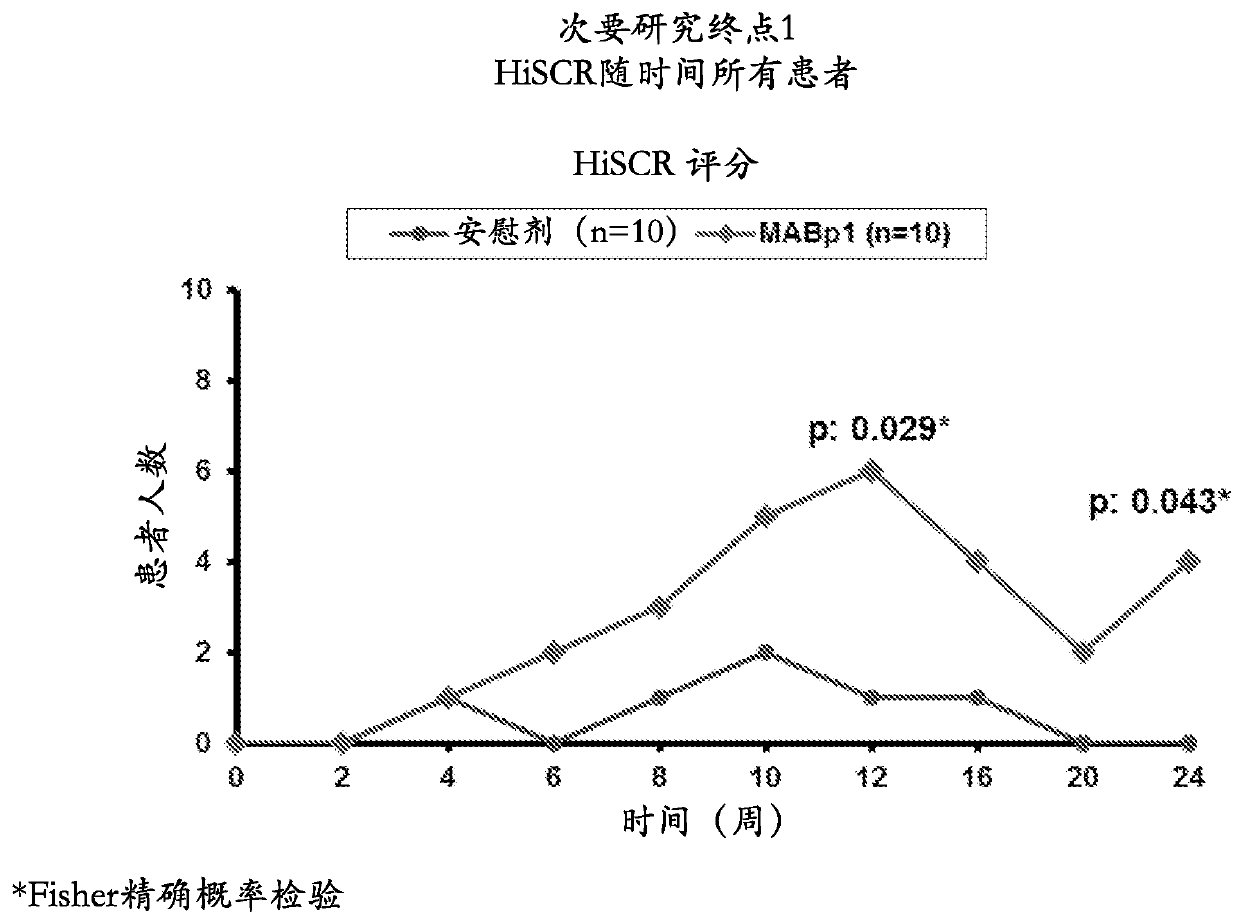
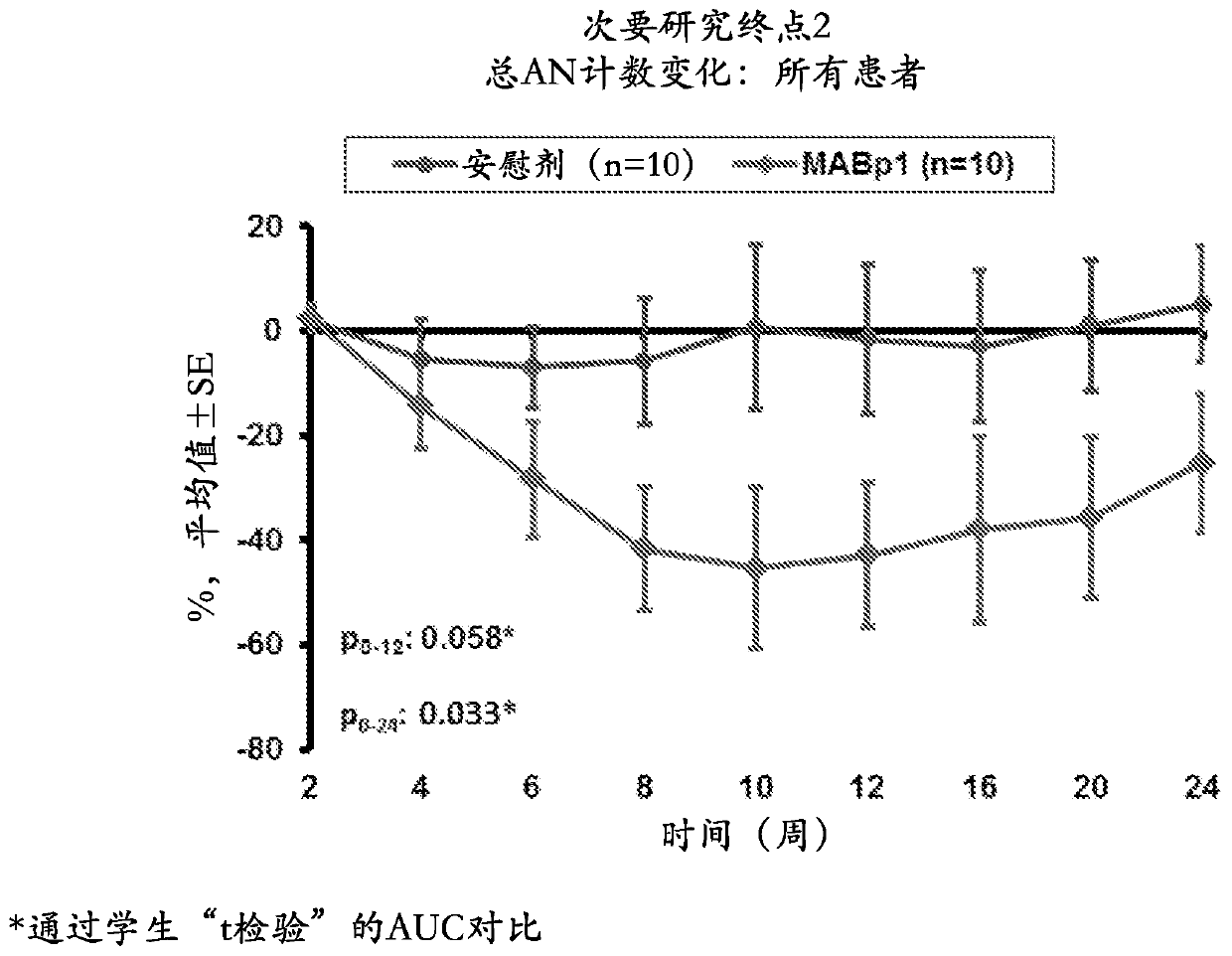
[0077] A 20-patient, double-blind, placebo-controlled study was designed to evaluate MABpl, a True Human drug targeting interleukin-1α (IL-1α) TM Antibody) safety and efficacy in HS patients ineligible for anti-TNFα therapy. Patients were randomized 1:1 to receive MABpl or placebo every 2 weeks for 12 weeks. Patients in the study underwent an initial efficacy assessment using the Hidradenitis Suppurativa Clinical Response (HiSCR) score at 12 weeks and continued with a follow-up phase to assess time to relapse 12 weeks after cessation of treatment. Outcome measures included assessment of the HiSCR score, a validated method for assessin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com