A detection protein with red fluorescent activity and its application
A red fluorescence and fluorescent protein technology, which is applied in the field of detection proteins with red fluorescence activity, can solve the problems of affecting stability and inactivation of biological macromolecules, and achieves the effect of simple preparation process and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The construction of embodiment 1 recombinant protein G
[0048] 1.1 Biomaterials
[0049] Escherichia coli BL21 was purchased from Nanjing Novizan Biotechnology Co., Ltd.; the plasmid pET-28a(+) was stored in the laboratory, and the bacterial liquid containing the C3 fragment[1] and the bacterial liquid containing the RFP sequence[2] were used for the experiment Room preservation.
[0050] [1] Xu Rui, Zhao Dengyun, Hong Yang, Lu Ke, Li Hao, Lin Jiaojiao, Feng Jintao, Xu Yumei, Zhu Chuangang. Domain remodeling, expression and identification of streptococcal protein G [J]. Chinese Journal of Animal Infectious Diseases ,2015,23(05):46-52.
[0051] [2] Kang Zeran. Cloning, prokaryotic expression characteristics and biological information analysis of monomeric red fluorescent protein gene DsRed2[D]. Yanbian University, 2016.
[0052] 1.2 Construction and synthesis of recombinant protein G gene sequence
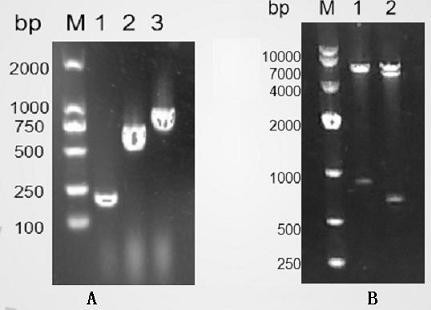
[0053] According to the gene sequence of the C3 fragment, the prime...
Embodiment 2
[0063] Expression and purification of embodiment 2 recombinant protein G
[0064] 2.1 Expression of recombinant plasmids
[0065] phase
[0066] (1) Transfer the pET-28a(+)-C3-RFP recombinant plasmids with correct identification results into BL21(DE3), inoculate them in 5ml LB liquid medium containing Kan+, and place them in a shaking incubator at 37°C. Shake culture at 250rpm.
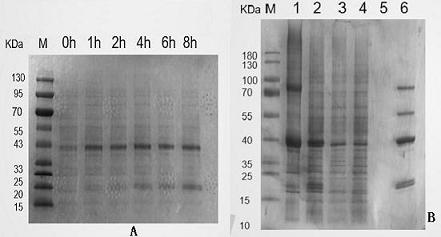
[0067] (2) (2) When growing to the logarithmic phase (OD600 is about 0.6), add IPTG with a final concentration of 1 mmol / L to induce expression. Take 0.5ml bacterial liquid before induction and 1h, 2h, 4h, 6h, 8h after induction, and analyze the best induction time by SDS-PAGE electrophoresis. ( image 3 A)
[0068] Massive expression:
[0069] (1) Transform the pET-28a(+)-C3-RFP recombinant plasmids with correct identification results into BL21(DE3), inoculate them in 150ml LB liquid medium containing Kan+, and place them in a shaking incubator at 37°C. Shake culture at 250rpm.
[0070] (2) W...
Embodiment 3
[0082] Example 3 Activity identification of C3-RFP recombinant protein
[0083] 3.1 Observation of fluorescence activity
[0084] Centrifuge the bacterial solution collected in the above phase at 10,000rpm for 5min, discard the supernatant, and add 200ul ddH 2 O resuspend the bacteria, after resuspension, pipette 10ul onto a clean glass slide, cover with a cover glass, excite with RFP excitation light under a fluorescent electron microscope, and observe whether the bacteria have red fluorescence. Fluorescence electron microscopy observations showed that ( Figure 4 ), the fluorescence of recombinant plasmid pET-28a(+)-C3-RFP successfully induced and expressed in E. coli BL21(DE3) increased with time at 1-8h, and the fluorescence reached the highest after 8h of induction and tended to be stable.
[0085] 3.2 Western blotting to detect the binding activity of recombinant protein and IgG
[0086] (1) Perform SDS-PAGE electrophoresis on the purified protein, then transfer the pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com