Selecting headache patients responsive to antibodies directed against calcitonin gene related peptide
A patient and antibody technology, applied in the direction of antibodies, antibody medical components, peptides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 5
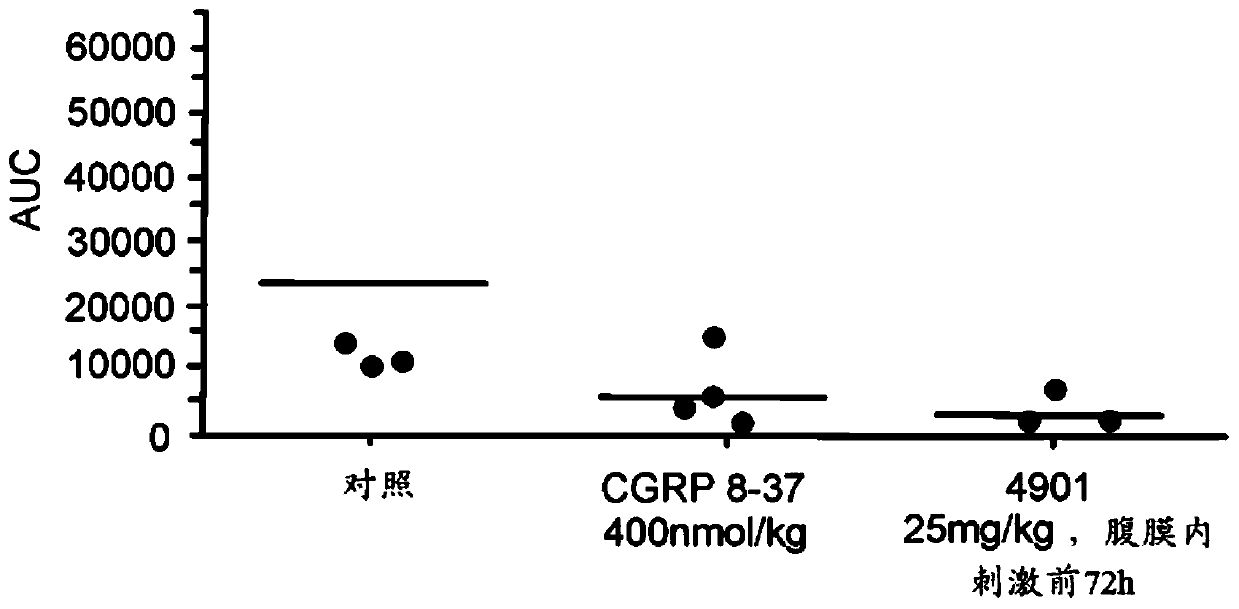
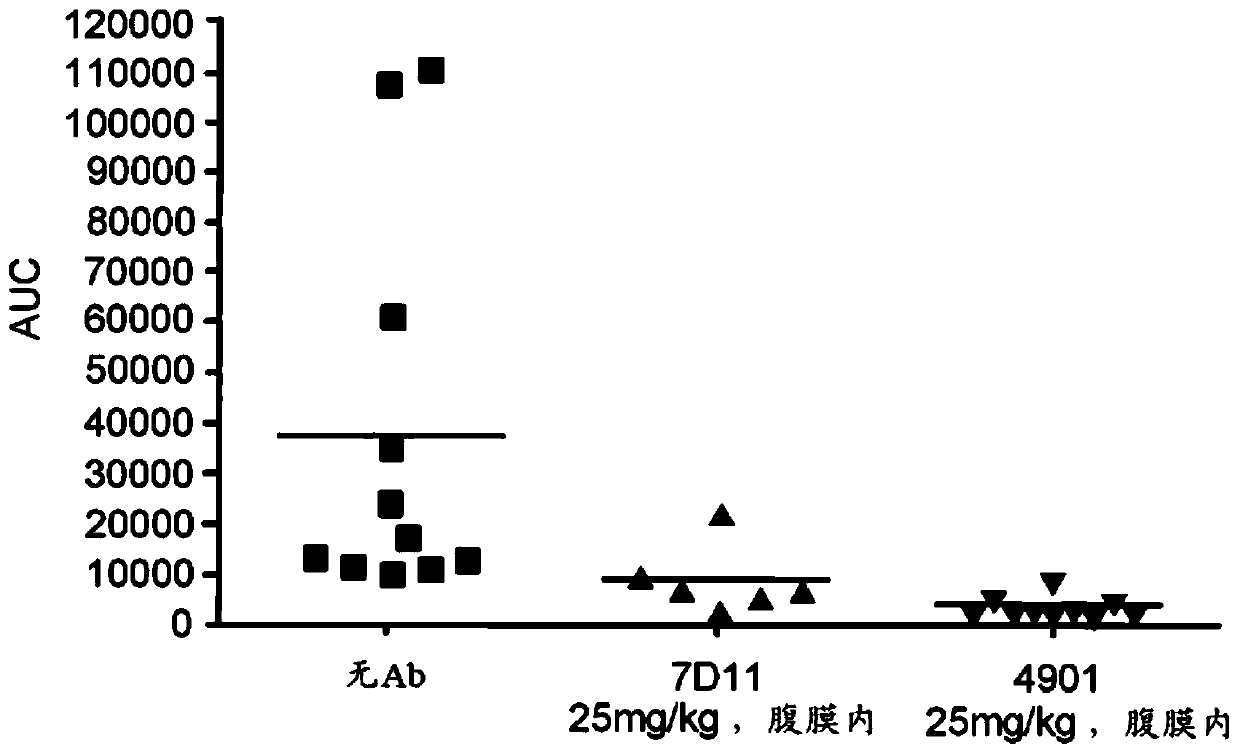
[0129] Example 5 describes the means that can be used to identify and select neurons (HT and WDR neurons) in rats. This example also describes observations made in conjunction with the activation and sensitization of each of these types of neurons following CSD induction.
[0130] Patients experiencing hyperalgesia may respond to a course of monoclonal antibodies that modulate (e.g., block, inhibit, suppress, or reduce) the CGRP pathway (e.g., longer anti-CGRP antibody courses and / or higher dose anti-CGRP antibody courses) A response, wherein the hyperalgesia is reduced (eg, reversed or eliminated) following administration of a monoclonal antibody that modulates (eg, blocks, inhibits, suppresses, or reduces) the CGRP pathway. If anti-CGRP antibodies reduce headache in hyperalgesic patients, then it is confirmed that the headache is mediated by HT neurons because, as shown in Example 5, anti-CGRP antibodies do not inhibit WDR, another class of nociceptive neurons. Example 6 de...
Embodiment 1
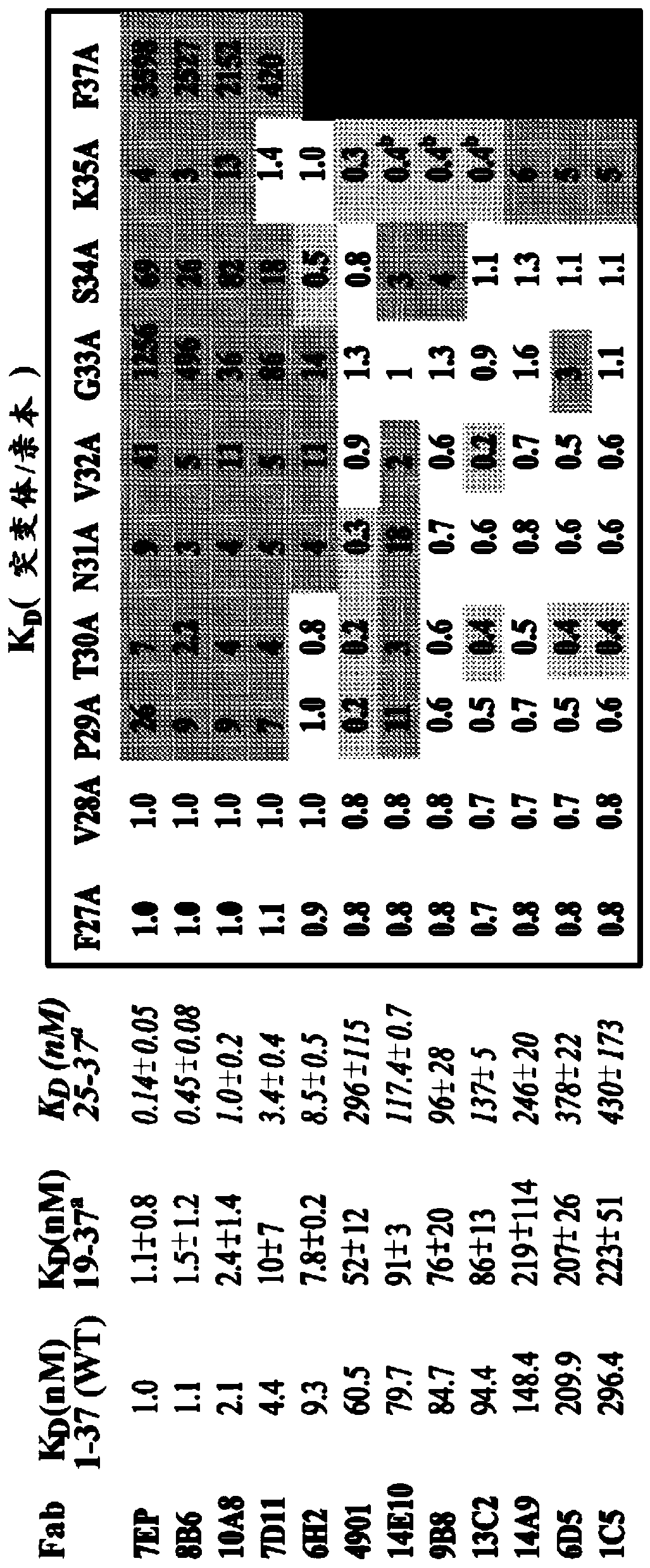
[0254] Example 1: Generation and Characterization of Monoclonal Antibodies Against CGRP
[0255] Generation of anti-CGRP antibodies. To generate anti-CGRP antibodies with cross-species reactivity to rat and human CGRP, adjuvants containing 25-100 μg of human α-CGRP or β-CGRP conjugated to KLH were administered at various time intervals Mice were immunized (50 μl per footpad, 100 μl total per mouse). Typically such as Geerligs HJ et al., 1989, J. Immunol. Methods 124:95-102; Kenney JS et al., 1989, J. Immunol. Methods 121:157-166; and Wicher K et al., 1989, Int. Arch. Allergy Appl. Immunizations were performed as described in Immunol. 89:128-135. Mice were first immunized with CFA (complete Freund's adjuvant) containing 50 μg of human α-CGRP or β-CGRP conjugated to KLH. After 21 days, human β-CGRP (for mice immunized for the first time with human α-CGRP) or α-CGRP (for mice immunized with human β-CGRP for the first ) IFA (incomplete Freund's adjuvant) to mice for the secon...
Embodiment 2
[0274] Example 2: Screening of anti-CGRP antagonist antibodies using in vitro assays.
[0275] Murine anti-CGRP antibodies were further screened for antagonist activity in vitro using cell-based cAMP activation assays and binding assays.
[0276] Antagonist activity measured by cAMP assay. Five microliters of human α-CGRP or rat α-CGRP (final concentration 50 nM) or rat α - CGRP or human α-CGRP (final concentration 0.1 nM-10 μM; as a positive control for c-AMP activation) was dispensed into 384-well plates (Nunc, Cat# 264657). Ten microliters of cells (human SK-N-MC if using human α-CGRP, or rat L6 from ATCC if using rat α-CGRP) in stimulation buffer (20 mM HEPES, pH 7.4 , 146mM NaCl, 5mM KCl, 1mM CaCl 2 , 1mM MgCl 2 and 500 μM 3-isobutyl-1-methylxanthine (IBMX)) were added to the wells of the plate. Plates were incubated at room temperature for 30 minutes.
[0277] After incubation, use HitHunter TM Enzyme Fragment Complementation Assay (Applied Biosystems) cAMP activ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com