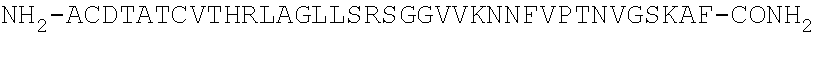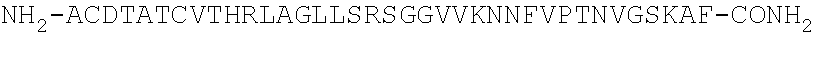Patents
Literature
63 results about "Calcitonin gene-related peptide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Calcitonin gene-related peptide (CGRP) is a member of the calcitonin family of peptides, which in humans exists in two forms: α-CGRP and β-CGRP, also known as CGRP I and CGRP II . α-CGRP is a 37-amino acid peptide and is formed from the alternative splicing of the calcitonin/CGRP gene located on chromosome 11. The less-studied β-CGRP differs in three amino acids (in humans) and is encoded in a separate gene in the same vicinity.
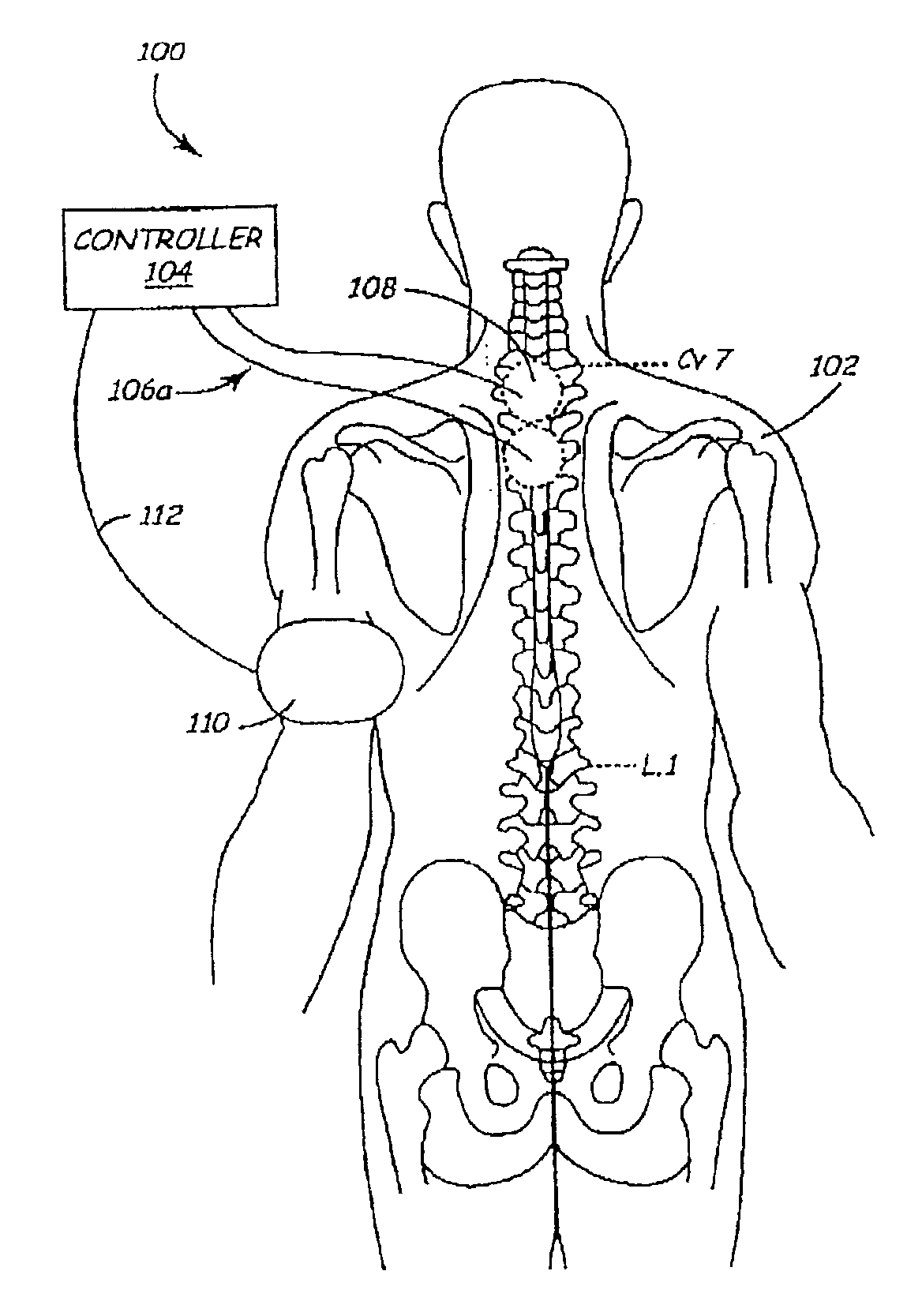
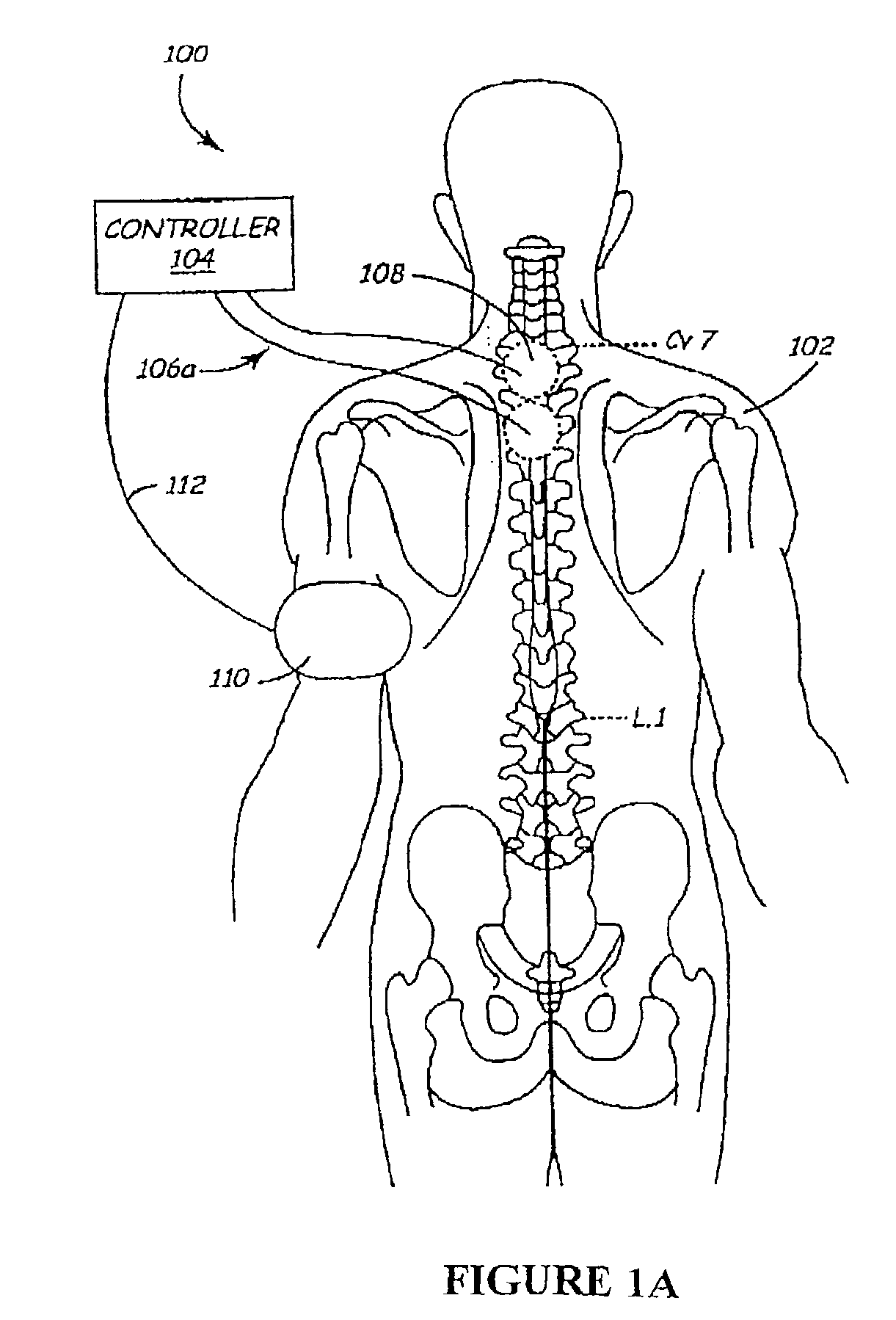
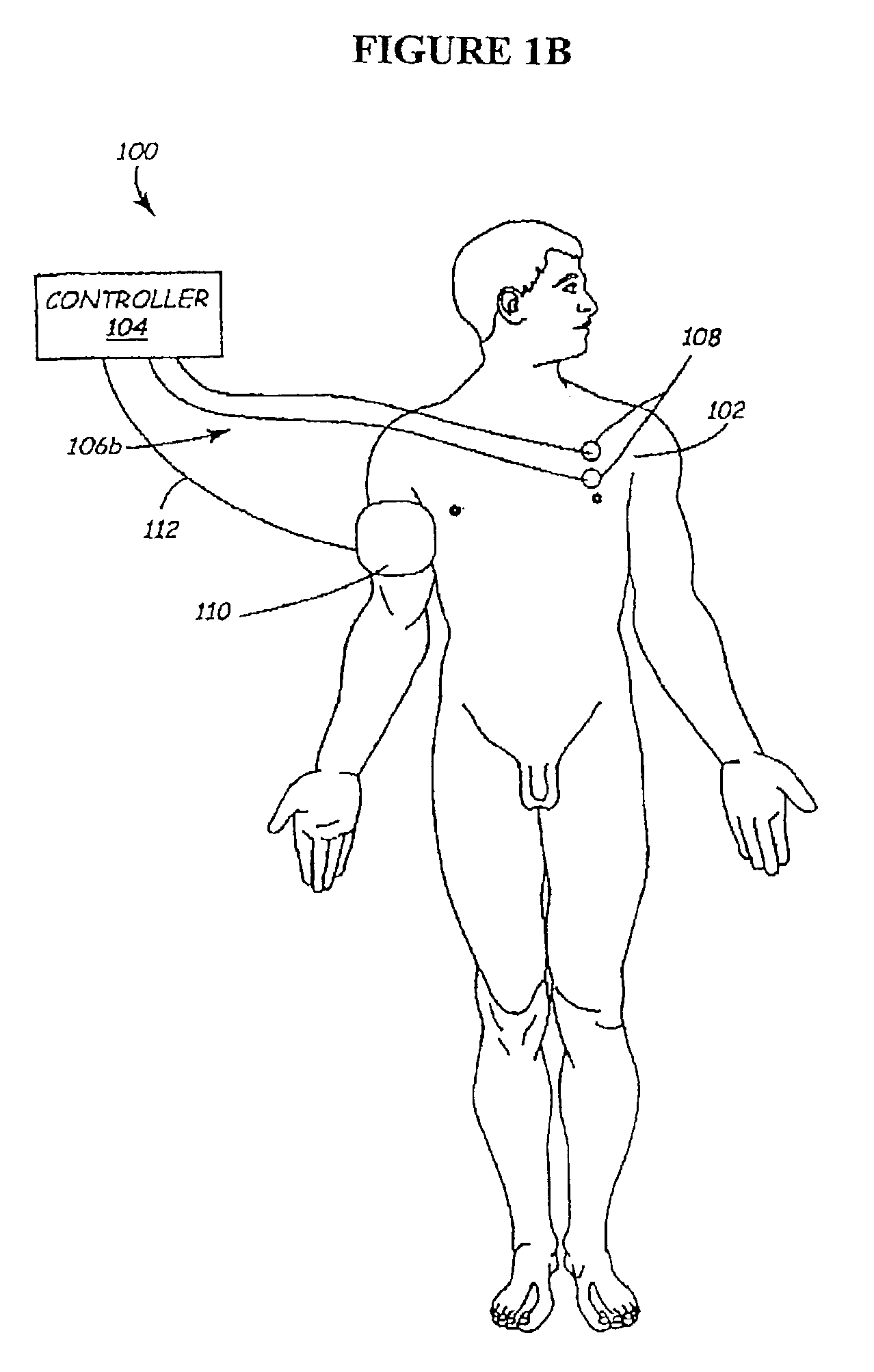
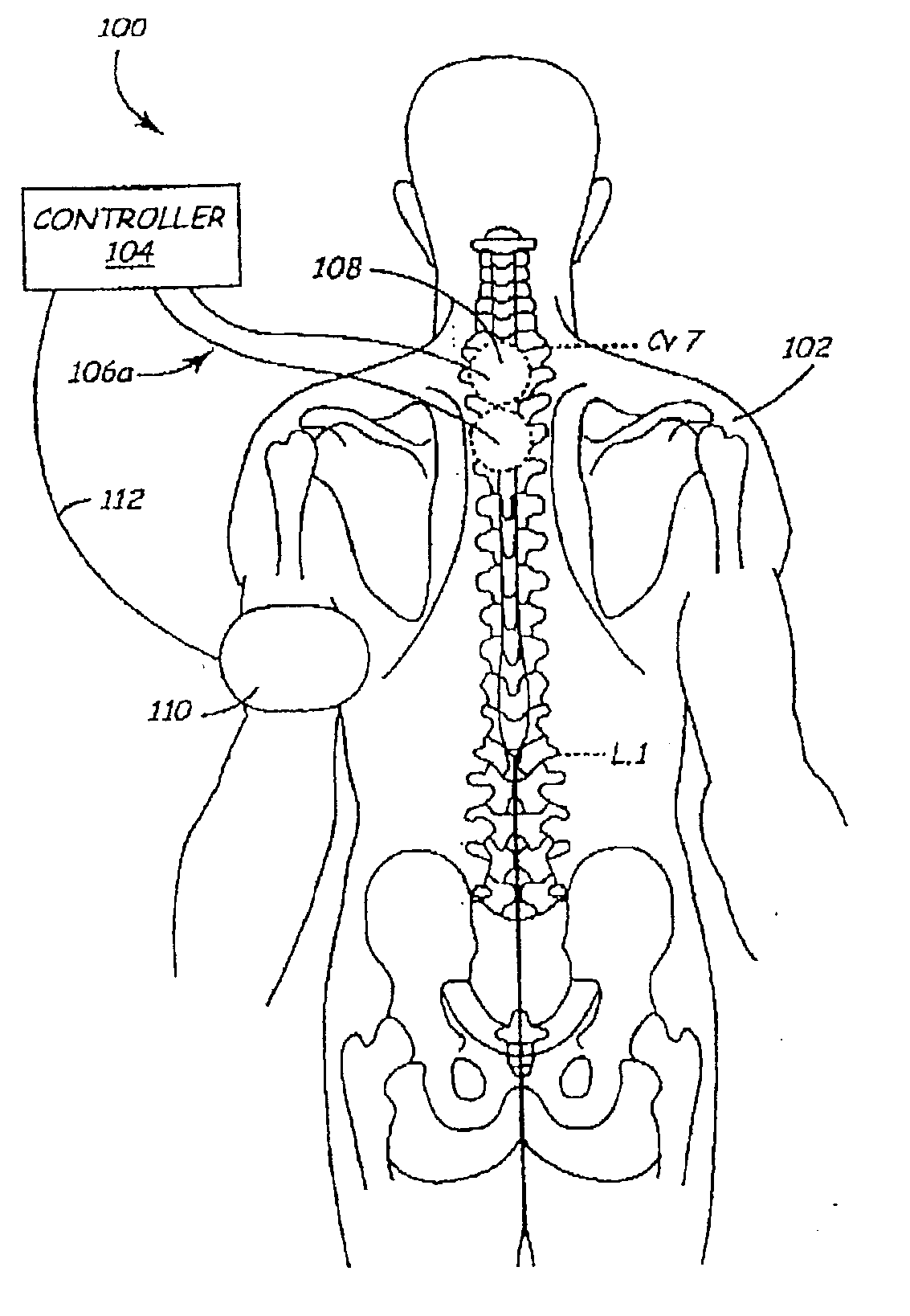
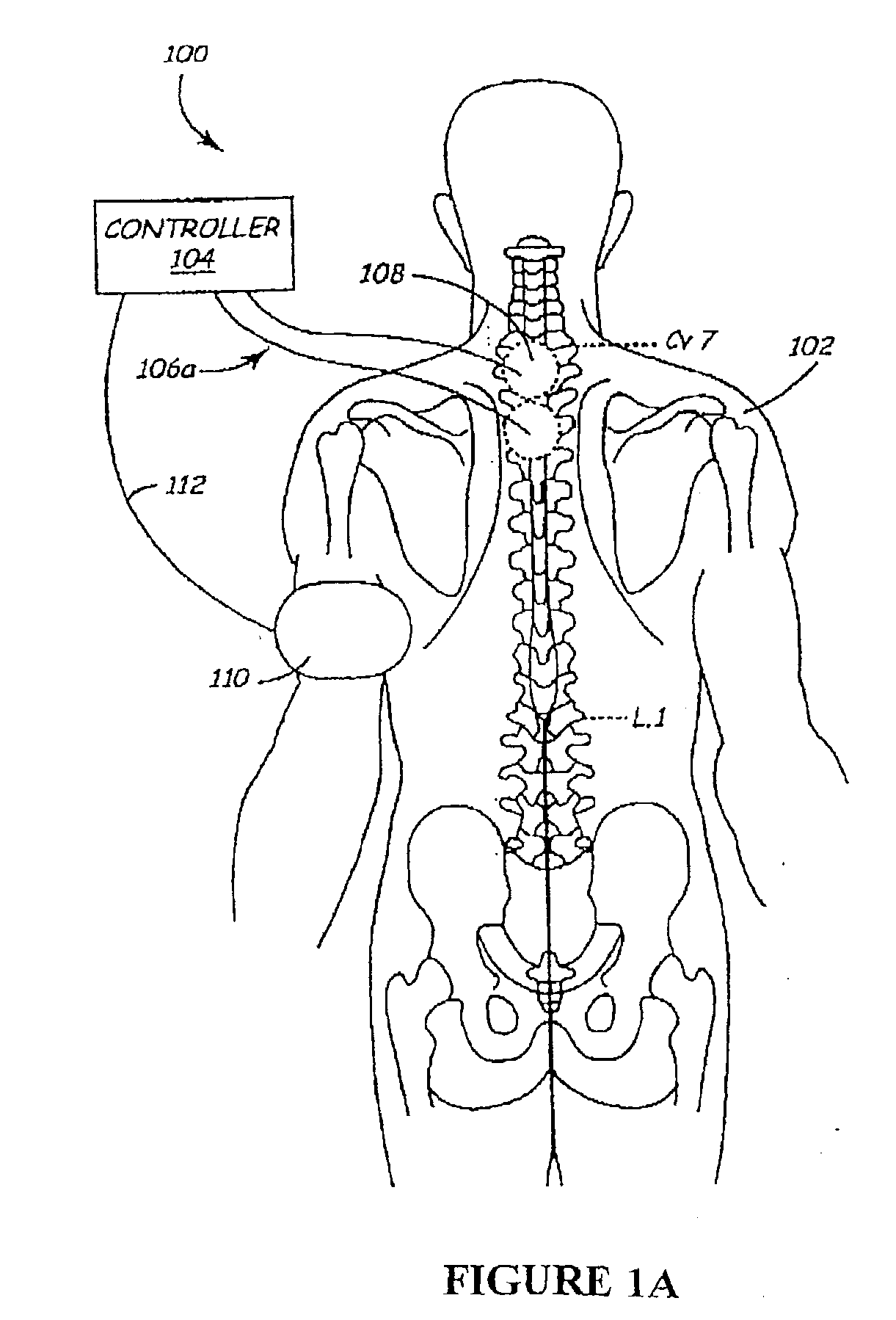
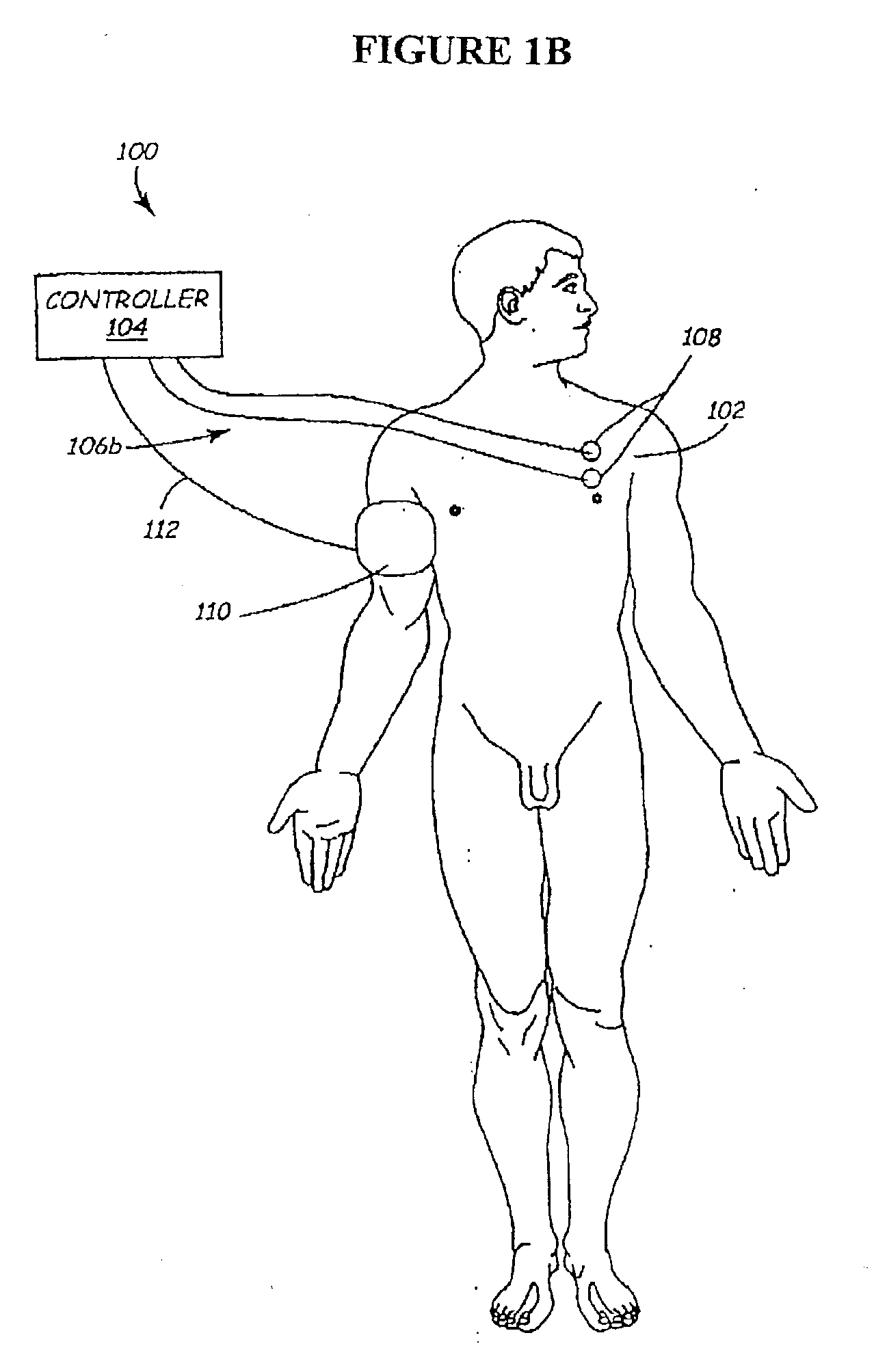
Methods and apparatus for the regulation of hormone release
ActiveUS7221979B2Modulate hormone levelPrevent hormone imbalancesInternal electrodesExternal electrodesCatecholamineElectrical stimulations
A method and apparatus for delivering corrective therapy through hormone regulation is provided. Inhibition of sympathetic fibers by spinal cord stimulation is used to regulate the levels of hormones such as catecholamines, renin, and calcitonin gene-related peptide. The invention utilizes a closed or open loop feedback system in which physiological parameters such as the concentrations of hormones and sympathetic indicators such as heart rate and urine production are monitored and used to determine the appropriate level of neurostimulation. The site of electrical stimulation includes, but is not limited to, the spinal cord at levels T7–L2 and the associated neural fibers within a region of the T7–L2 dermatomes
Owner:MEDTRONIC INC
Methods and apparatus for the regulation of hormone release
A method and apparatus for delivering corrective therapy through hormone regulation is provided. Inhibition of sympathetic fibers by spinal cord stimulation is used to regulate the levels of hormones such as catecholamines, renin, and calcitonin gene-related peptide. The invention utilizes a closed or open loop feedback system in which physiological parameters such as the concentrations of hormones and sympathetic indicators such as heart rate and urine production are monitored and used to determine the appropriate level of neurostimulation. The site of electrical stimulation includes, but is not limited to, the spinal cord at levels T7-L2 and the associated neural fibers within a region of the T7-L2 dermatomes
Owner:MEDTRONIC INC
Therapeutic/cosmetic compositions comprising CGRP antagonists for treating sensitive human skin
Topically applicable pharmaceutical / dermatological / cosmetic compositions well suited for the therapeutic treatment or care of sensitive human skin, hair, mucous membranes, nails and / or the scalp, in particular for reducing or avoiding the skin-irritant side effects of a variety of bioactive agents, for example the alpha -hydroxy acids, comprise a therapeutically / cosmetically effective amount of at least one calcitonin gene related peptide ("CGRP") antagonist, e.g., CGRP 8-37 or an anti-CGRP antibody.
Owner:LOREAL SA
Method of biochemical treatment of persistent pain
InactiveUS20050152905A1Reduce releaseAvoid exposureBiocidePeptide/protein ingredientsInterleukin 6Interleukin-1beta
This invention relates to a method for the biochemical treatment of persistent pain disorders by inhibiting the biochemical mediators of inflammation in a subject comprising administering to said subject any one of several combinations of components that are inhibitors of biochemical mediators of inflammation. Said process for biochemical treatment of persistent pain disorders is based on Sota Omoigui's Law, which states: ‘The origin of all pain is inflammation and the inflammatory response’. Sota Omoigui's Law of Pain unifies all pain syndromes as sharing a common origin of inflammation and the inflammatory response. The various biochemical mediators of inflammation are present in differing amounts in all pain syndromes and are responsible for the pain experience. Classification and treatment of pain syndromes should depend on the complex inflammatory profile. A variety of mediators are generated by tissue injury and inflammation. These include substances produced by damaged tissue, substances of vascular origin as well as substances released by nerve fibers themselves, sympathetic fibers and various immune cells. Biochemical mediators of inflammation that are targeted for inhibition include but are not limited to: prostaglandin, nitric oxide, tumor necrosis factor alpha, interleukin 1-alpha, interleukin 1-beta, interleukin-4, Interleukin-6 and interleukin-8, histamine and serotonin, substance P, Matrix Metallo-Proteinase, calcitonin gene-related peptide, vasoactive intestinal peptide as well as the potent inflammatory mediator peptide proteins neurokinin A, bradykinin, kallidin and T-kinin.
Owner:OMOIGUI OSEMWOTA SOTA
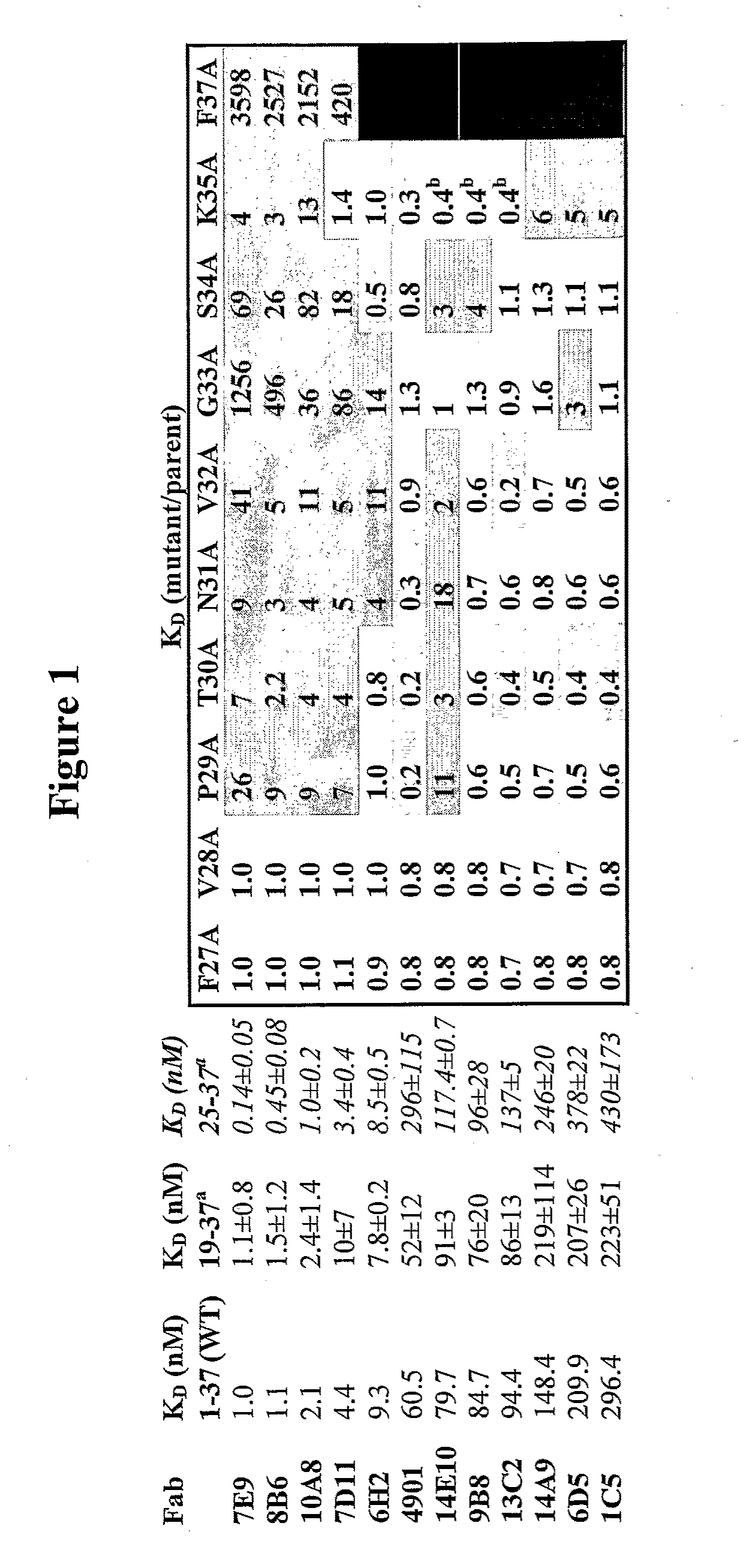
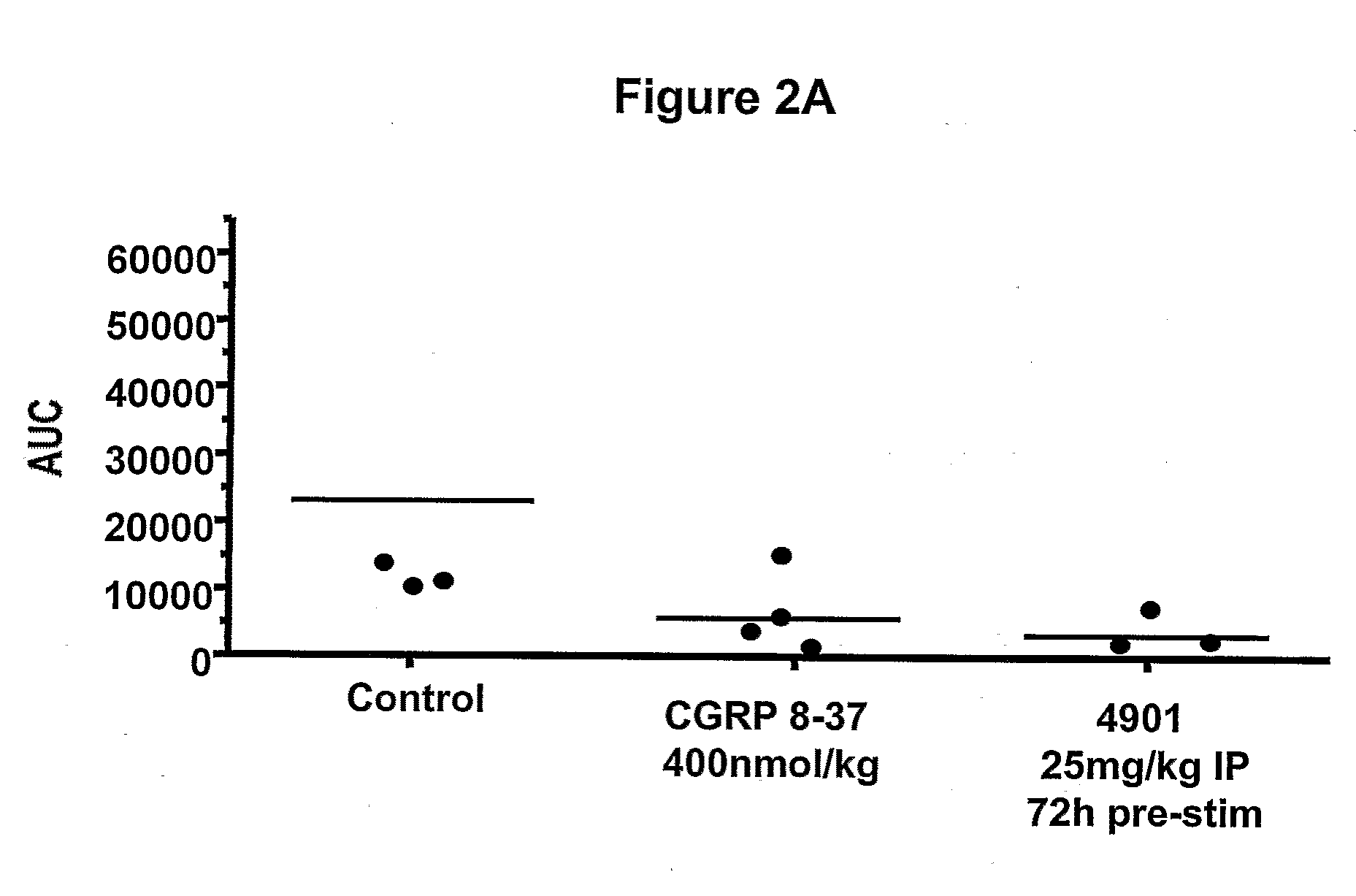
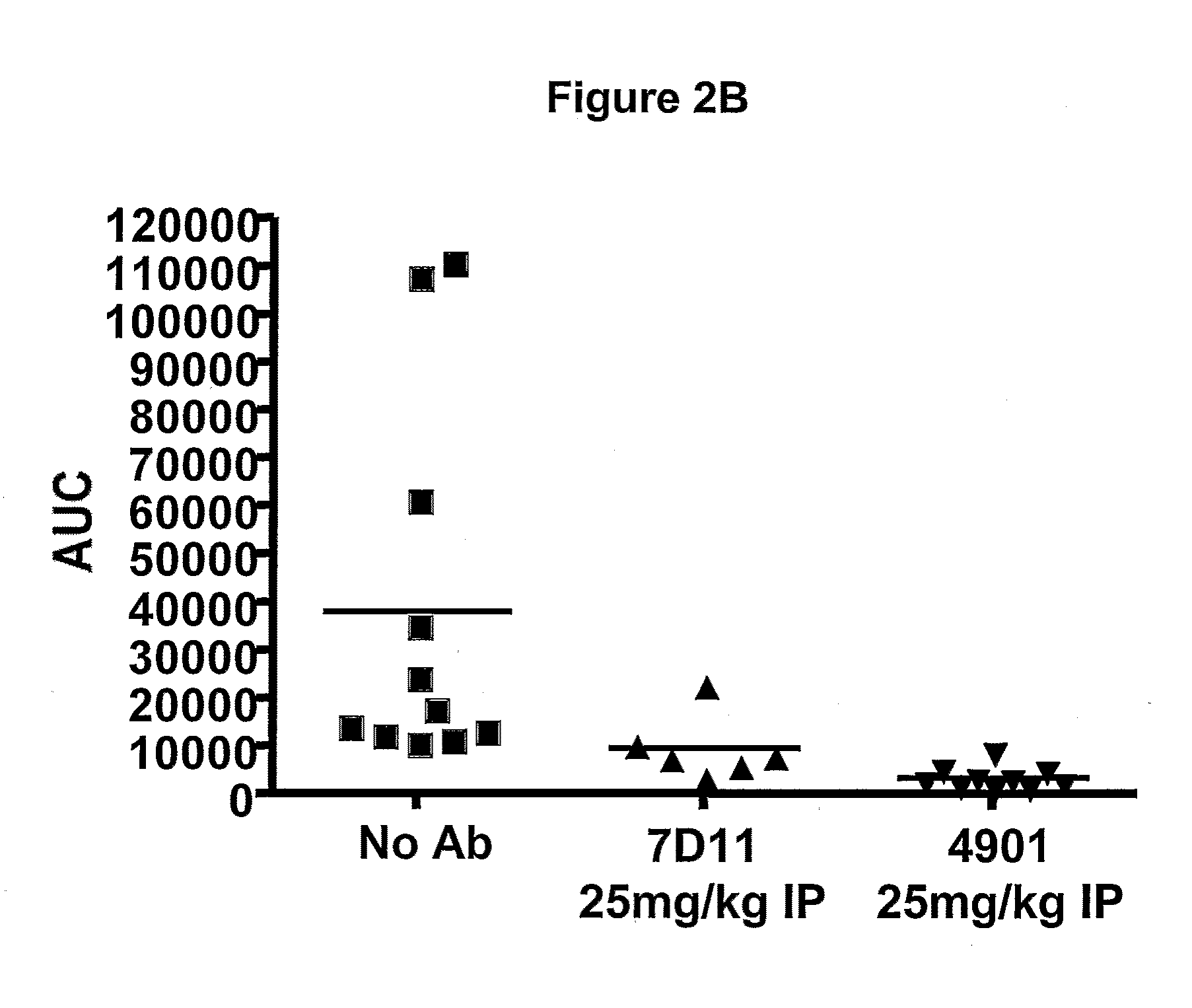
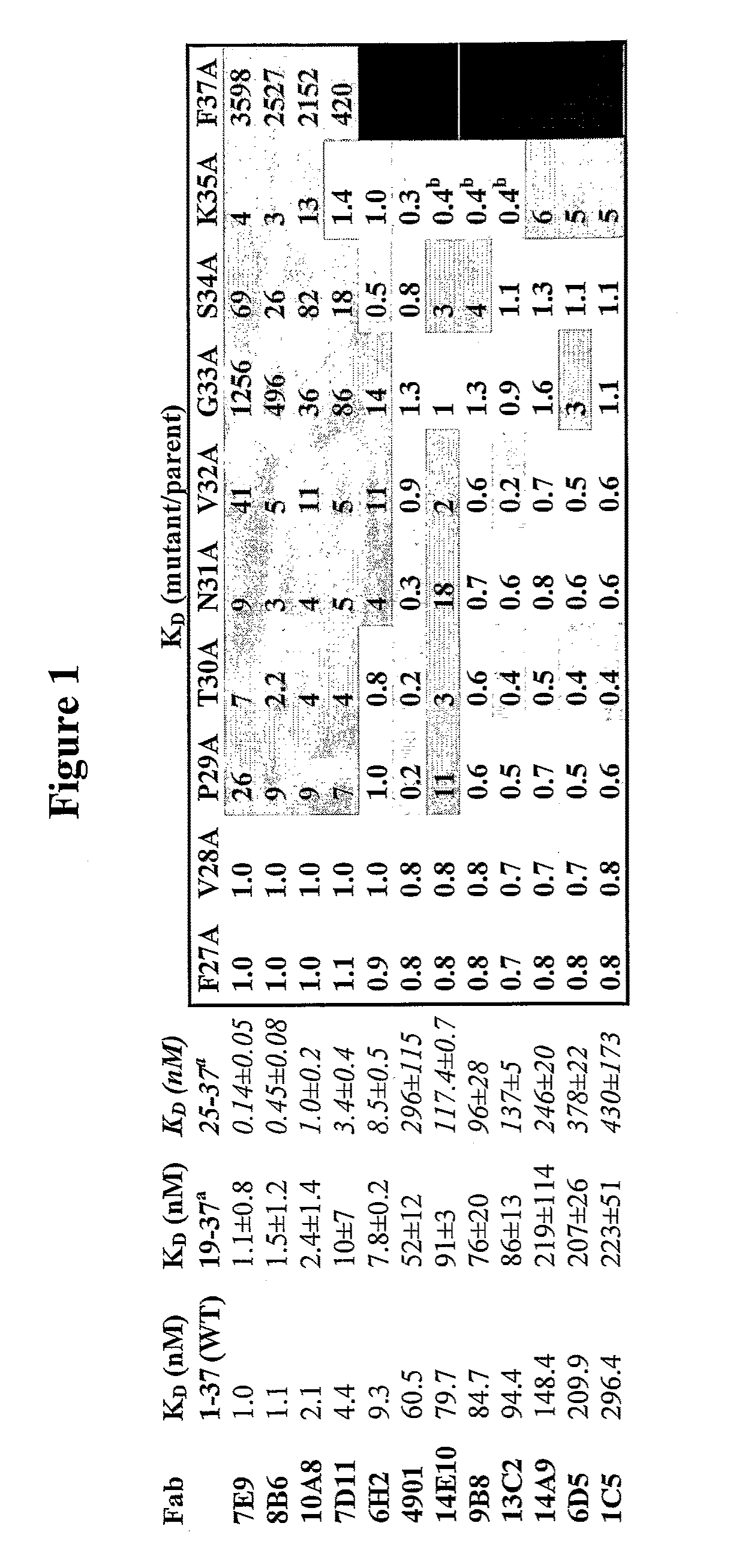
Antagonist antibodies directed against calcitonin gene-related peptide and methods using same
ActiveUS20090220489A1Reduce developmentDelay progressOrganic active ingredientsNervous disorderVasomotor symptomCluster headache
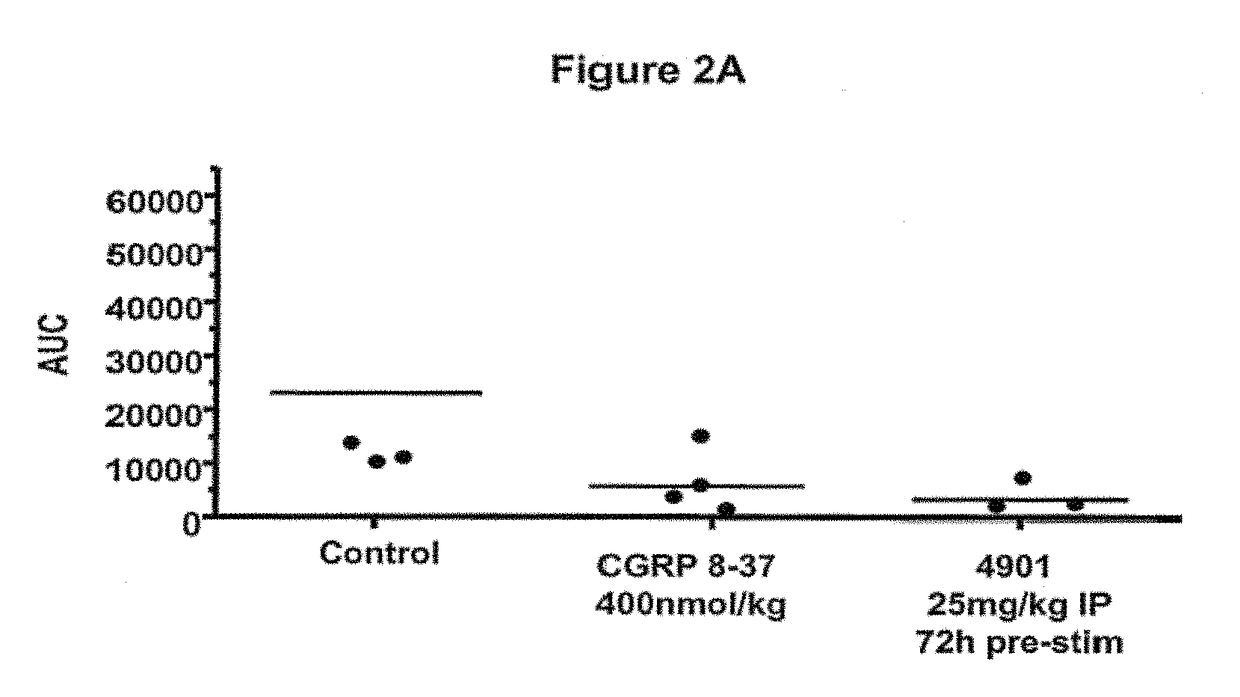
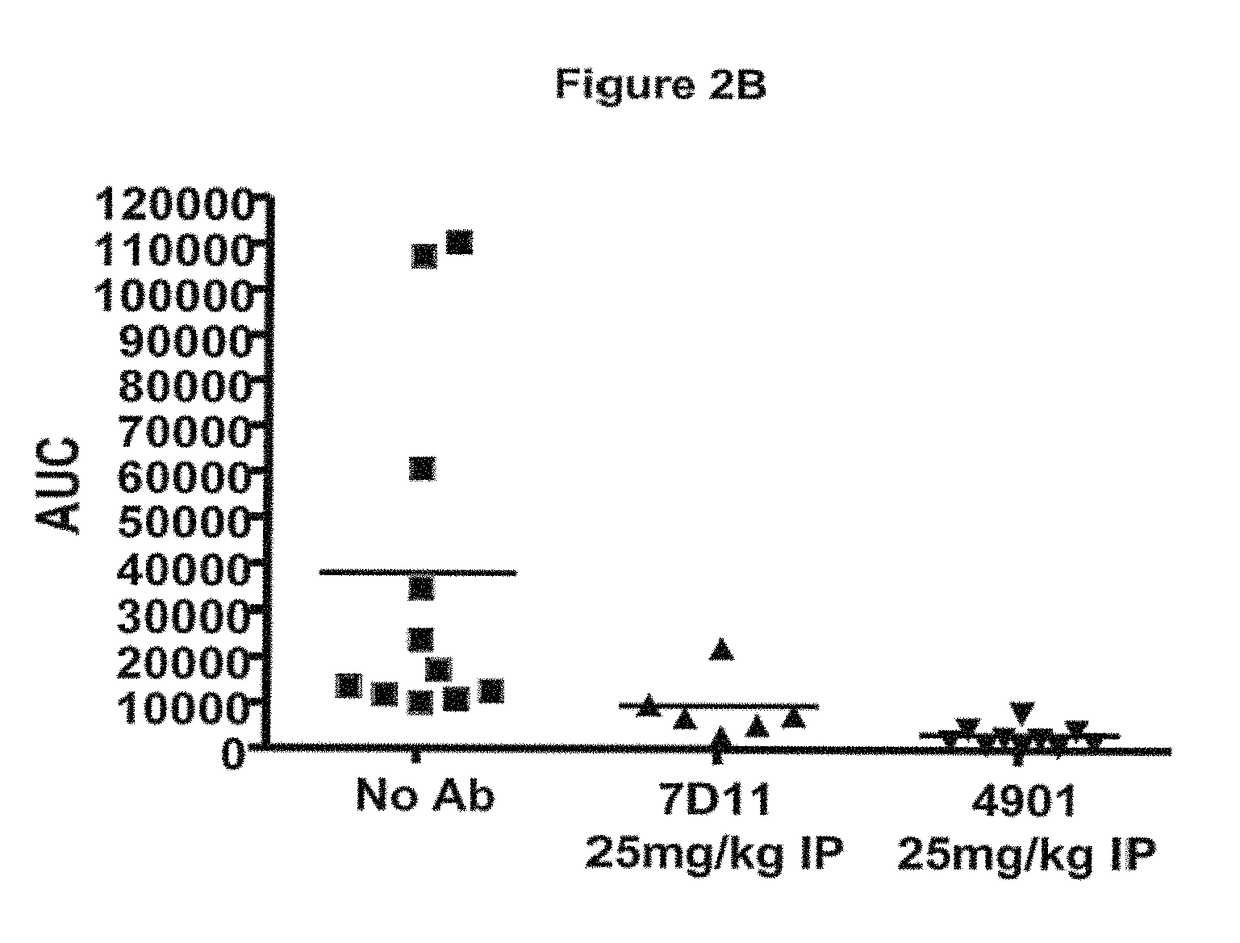
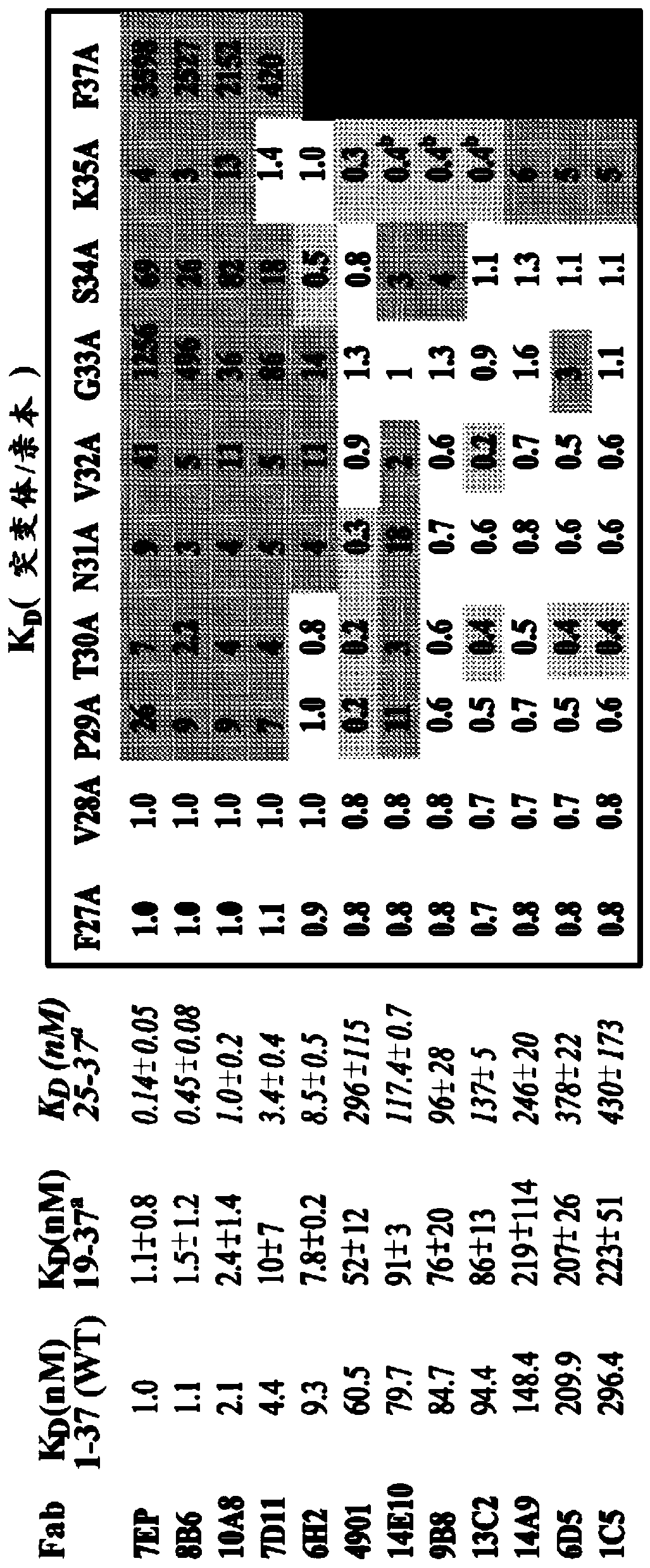
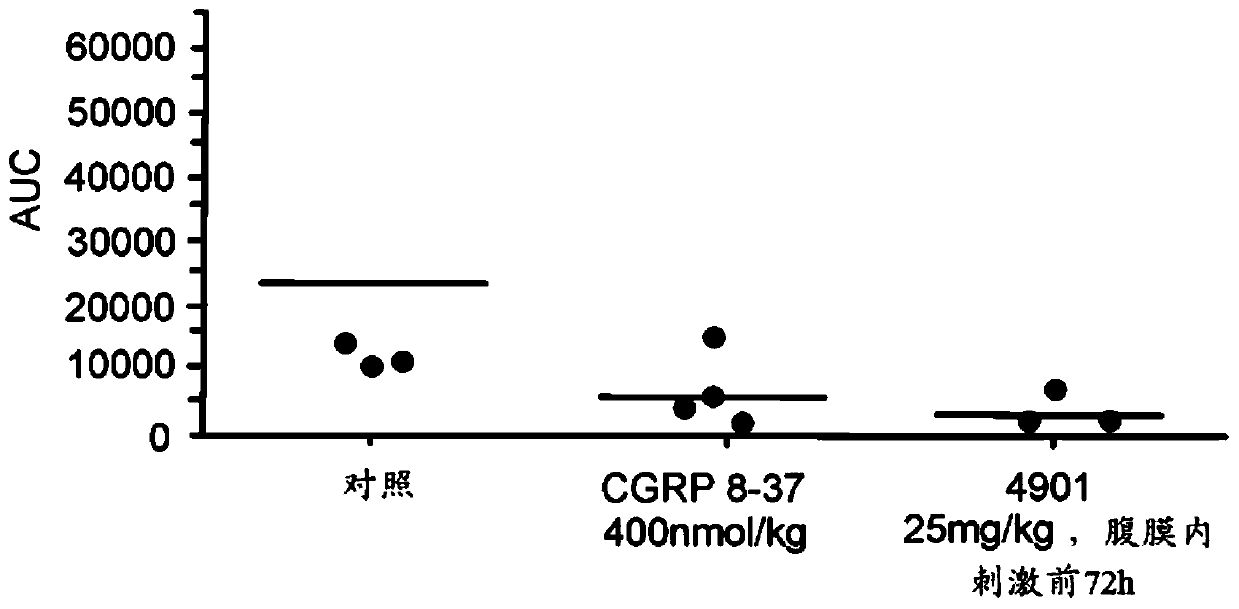
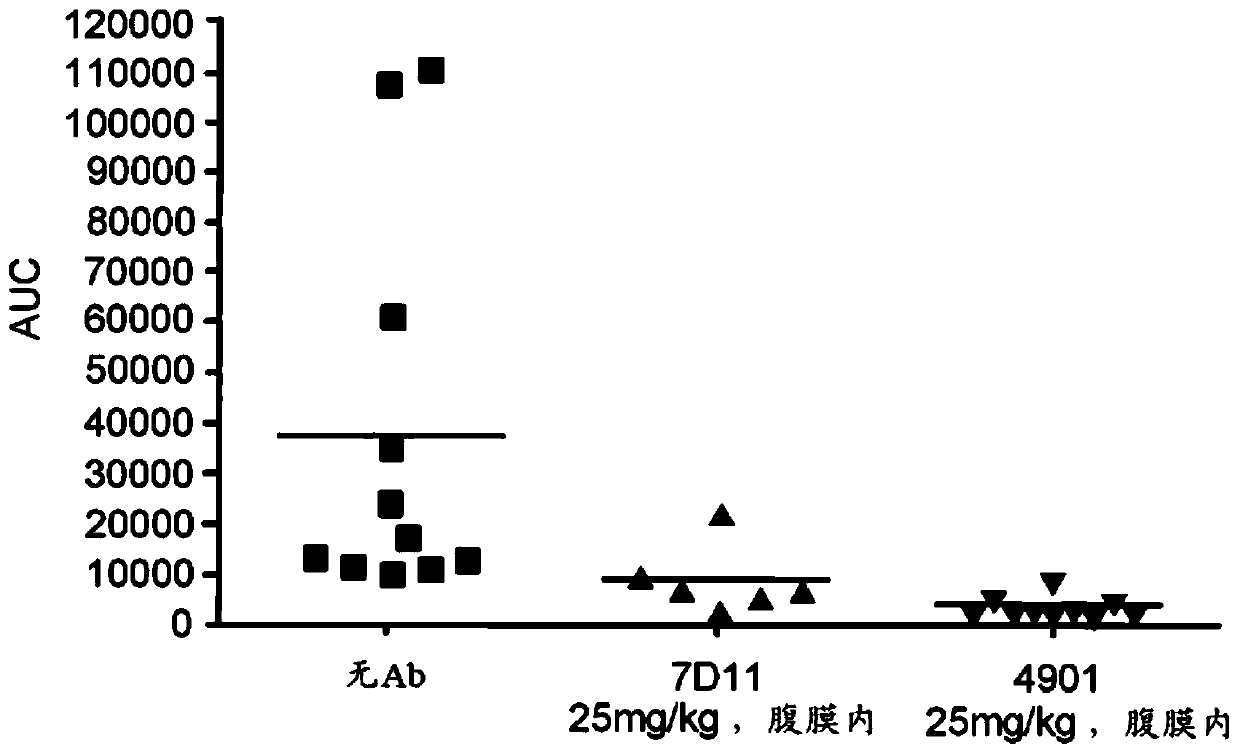
The invention features methods for preventing or treating CGRP associated disorders such as vasomotor symptoms, including headaches (e.g., migraine, cluster headache, and tension headache) and hot flushes, by administering an anti-CGRP antagonist antibody. Antagonist antibody G1 and antibodies derived from G1 directed to CGRP are also described.
Owner:TEVA PHARMACEUTICALS INTERNATIONAL GMBH
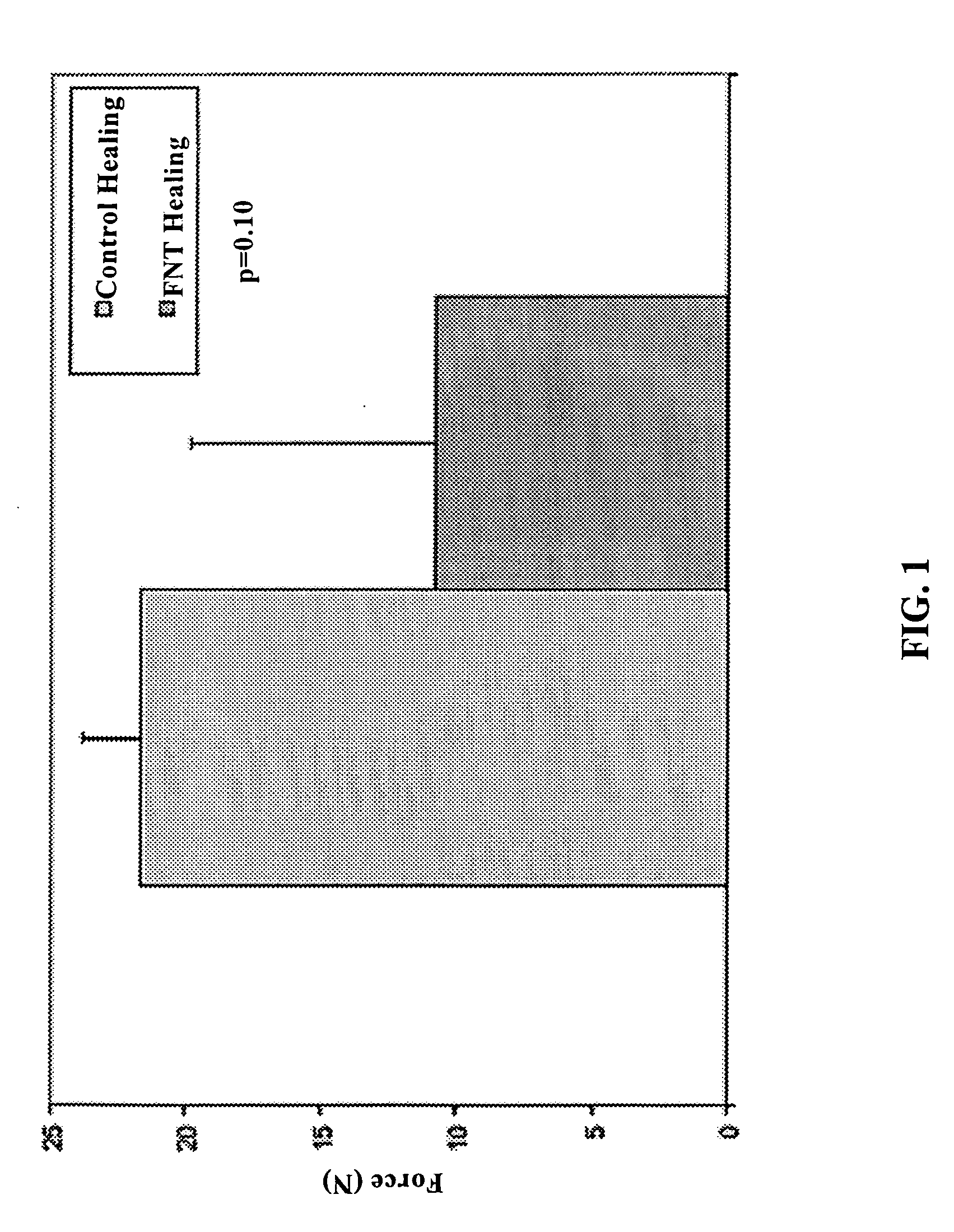
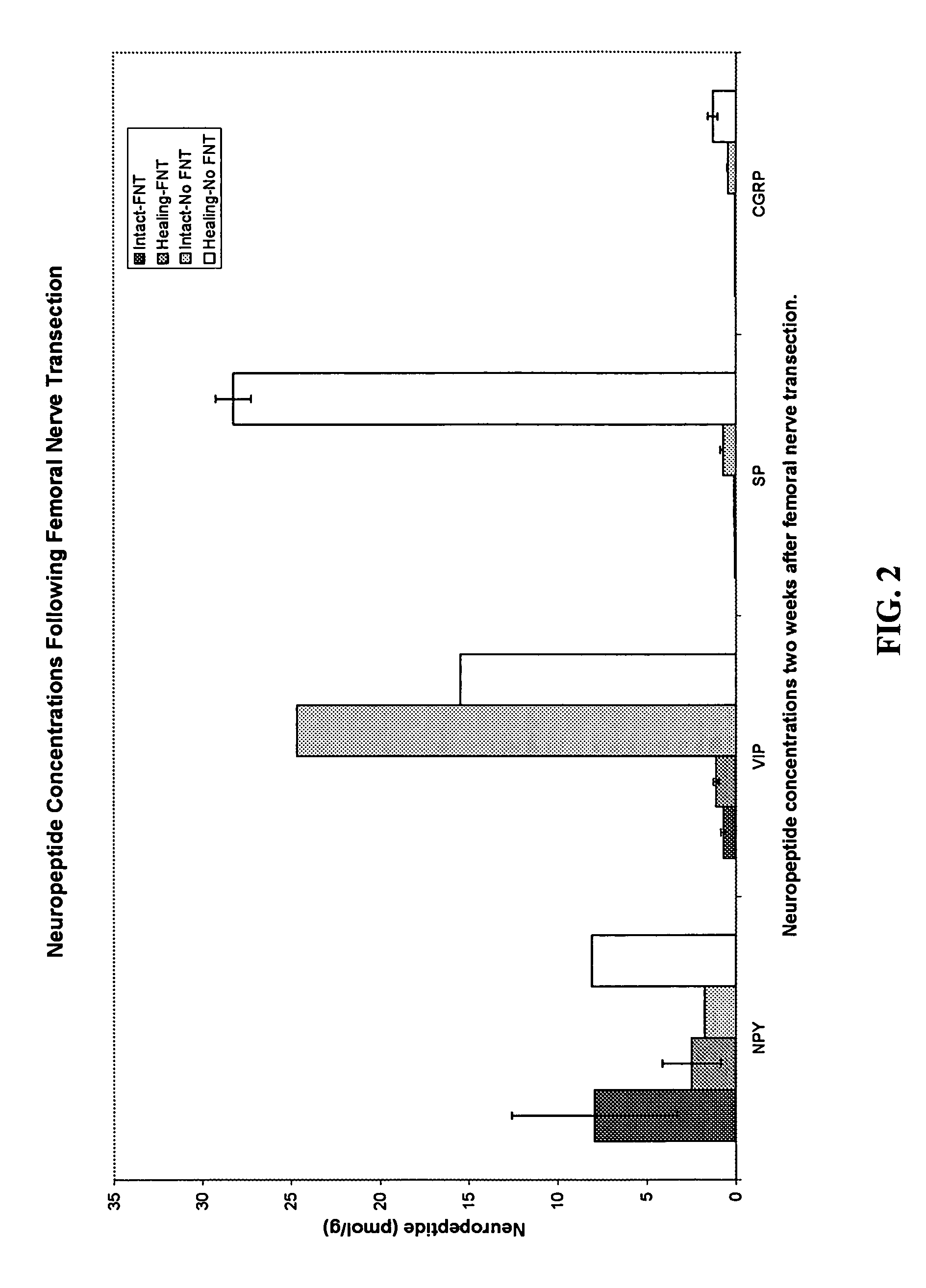
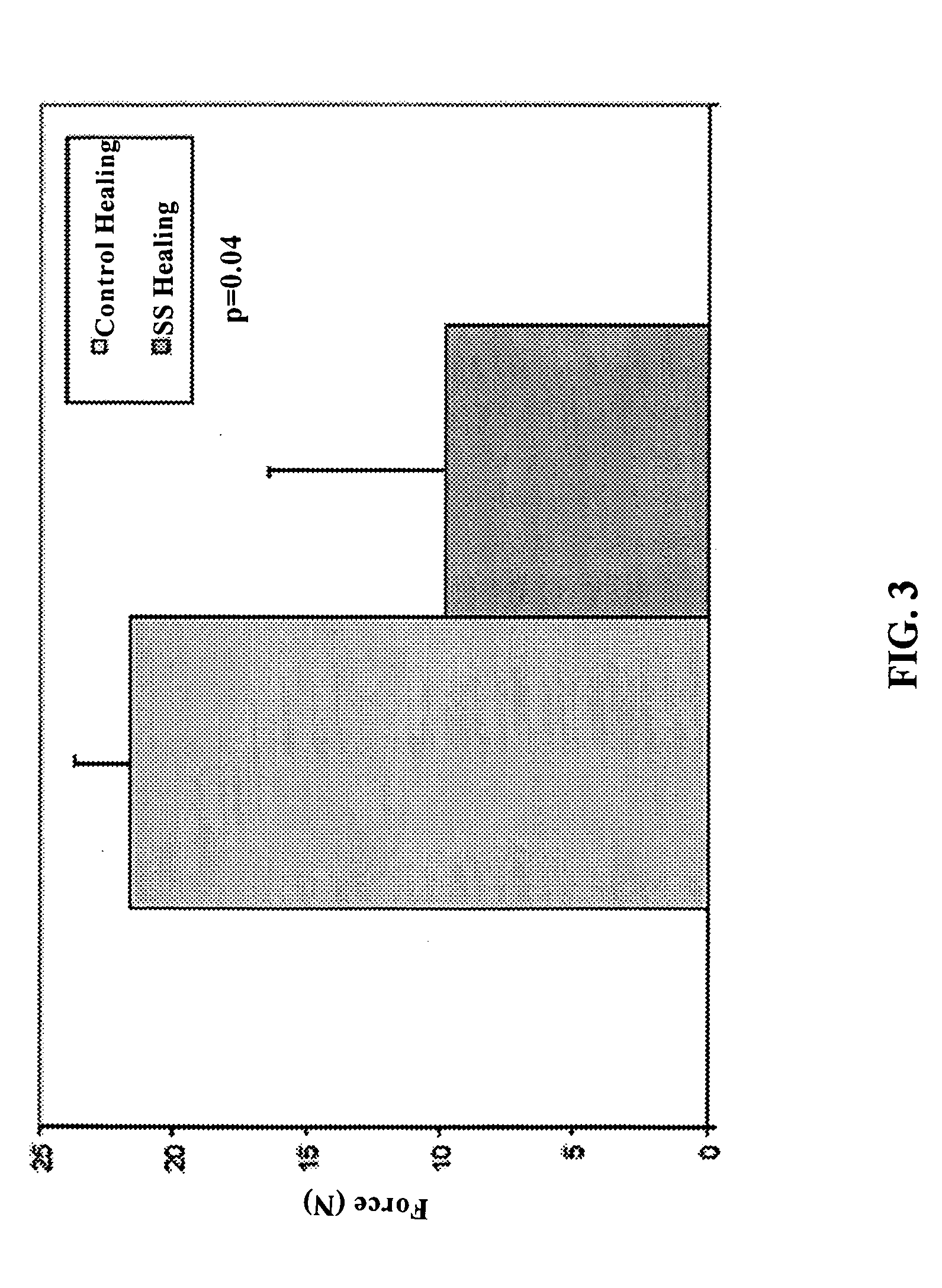
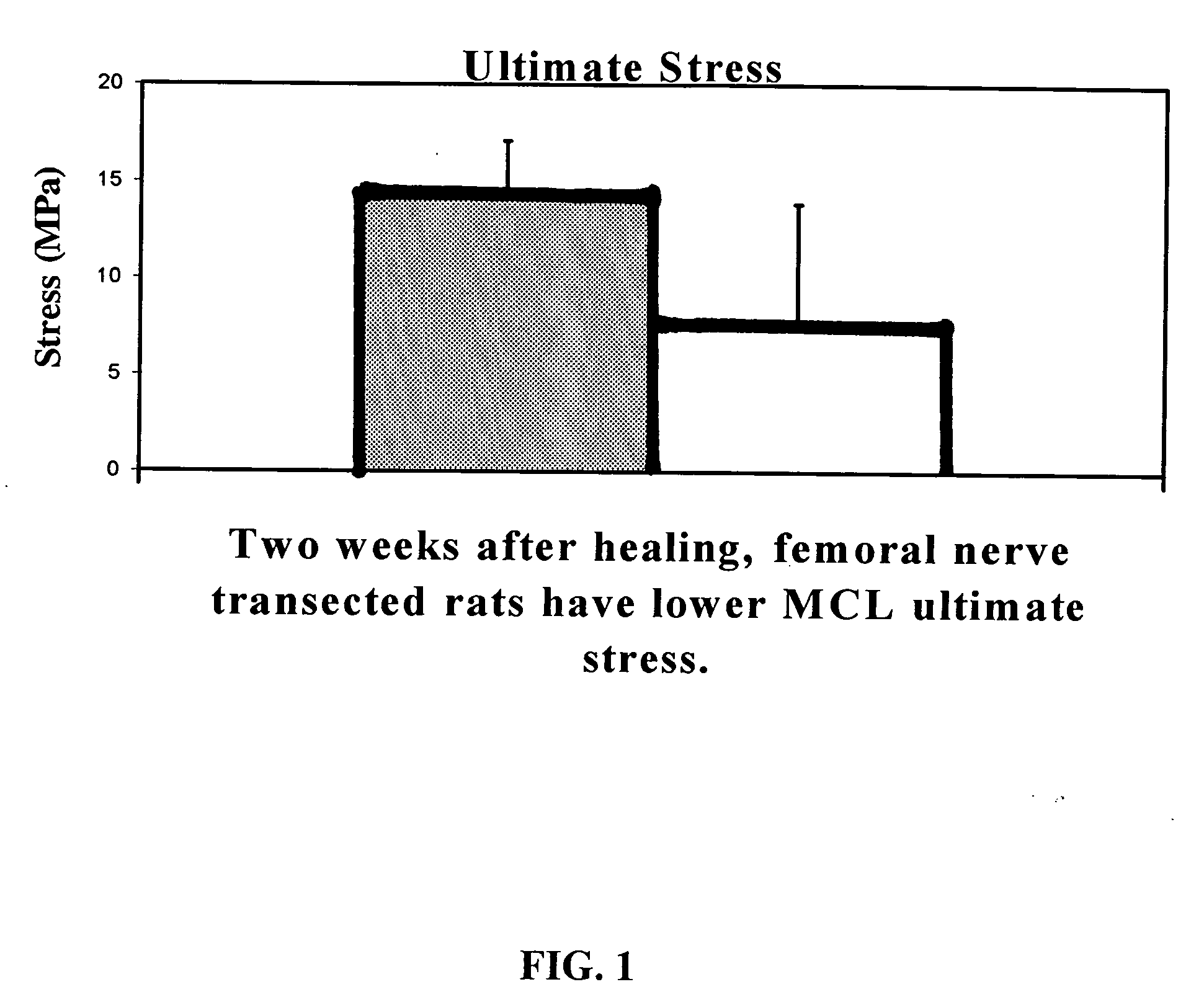
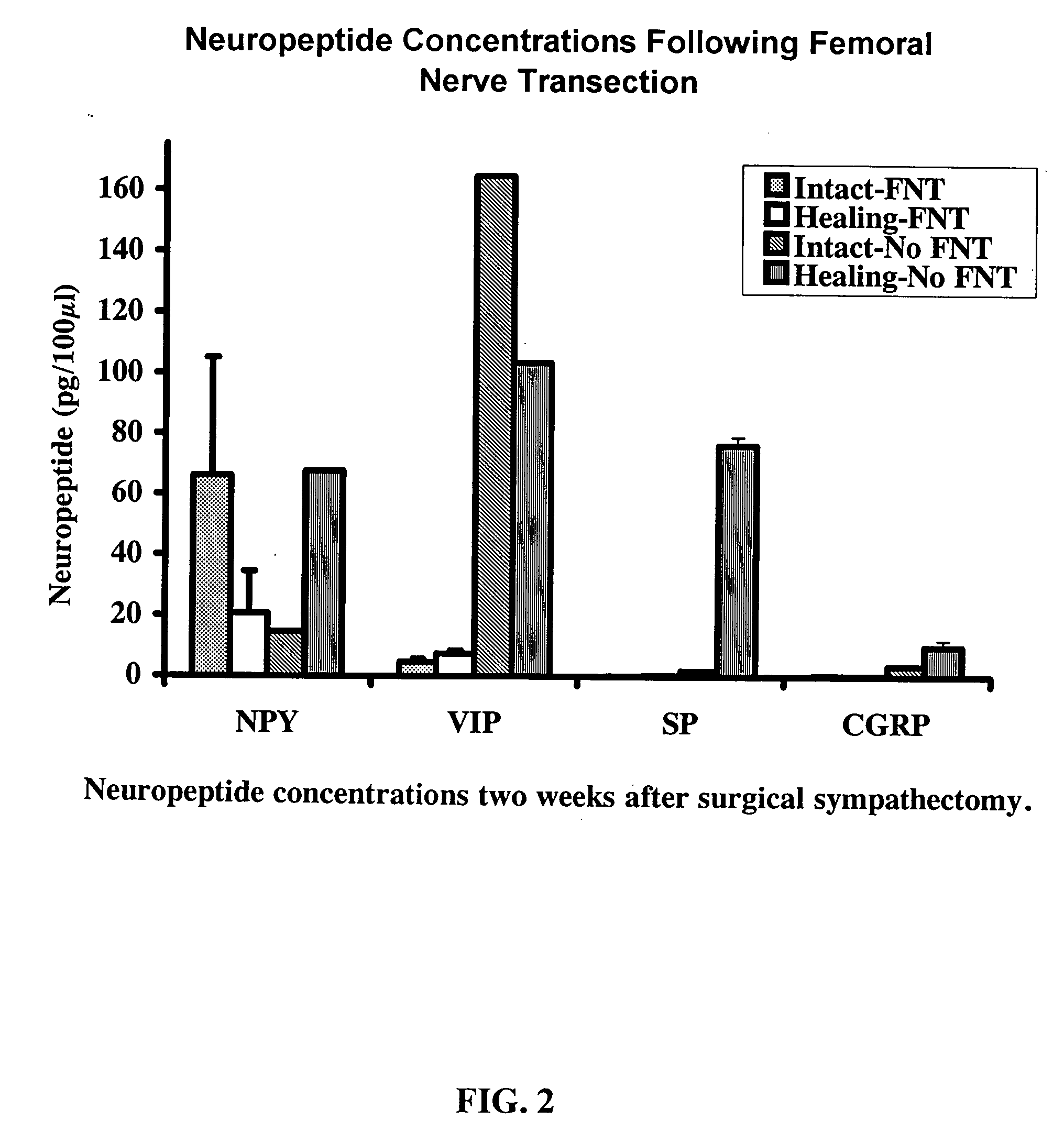
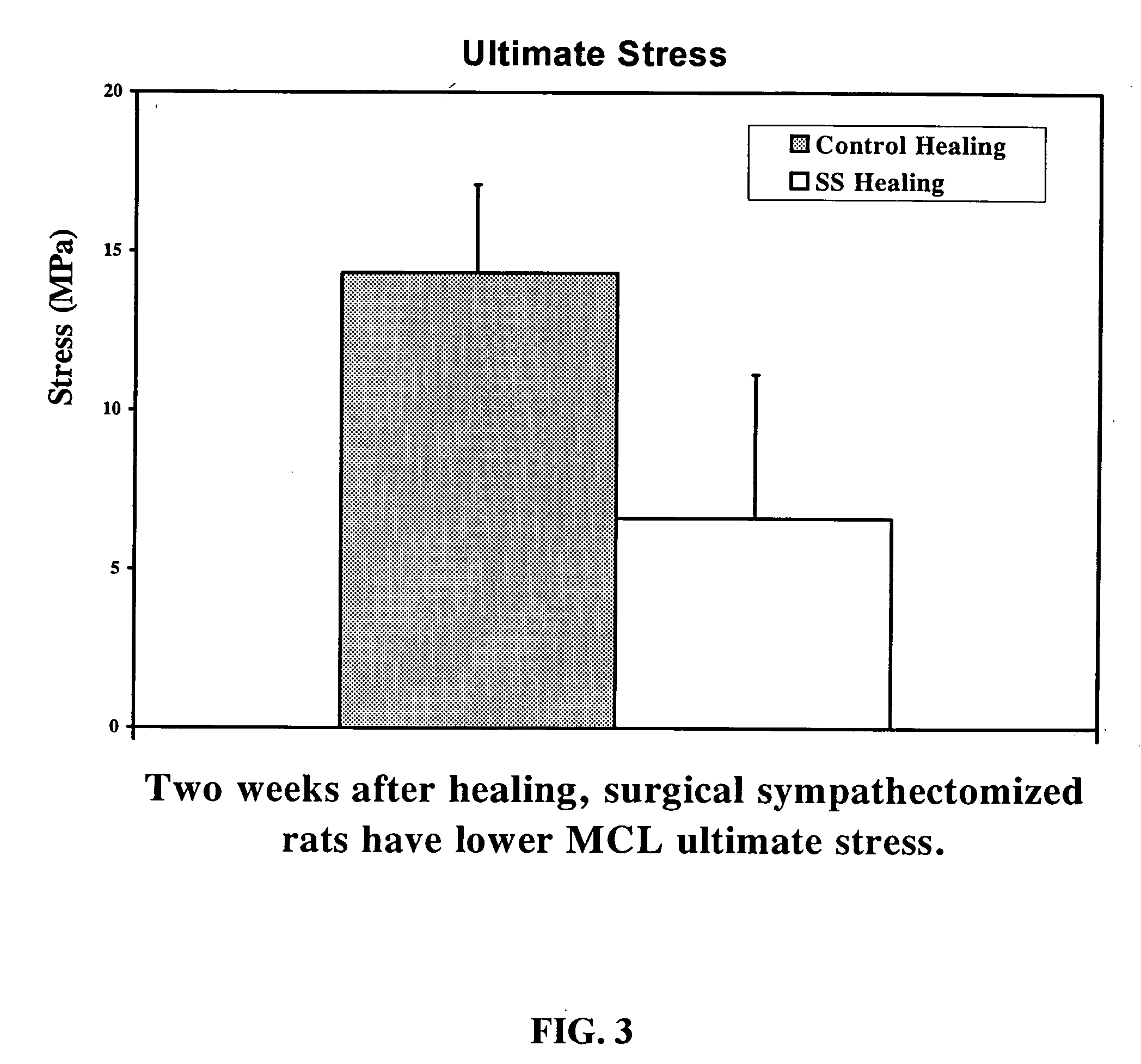
Use of neuropeptides for ligament, cartilage, and bone healing
Disclosed are a method and a corresponding pharmaceutical composition for treating damaged cartilage and subchondral bone. Neurogenic compounds in general and neuropeptides in particular have been found to be highly effective in stimulated repair of cartilage and bone damaged due to traumatic injury, ligament disease, and disuse. Preferred active ingredients for use in the method and corresponding pharmaceutical composition include calcitonin gene-related peptide (CGRP), cholecystokinin (CCK), dynorphin, enkephalin, galanin, neuropeptide Y (NPY), neurotensin, somatostatin, substance P (SP), thyrotropin-releasing hormone (TRH), vasoactive intestinal peptide (VIP).
Owner:WISCONSIN ALUMNI RES FOUND
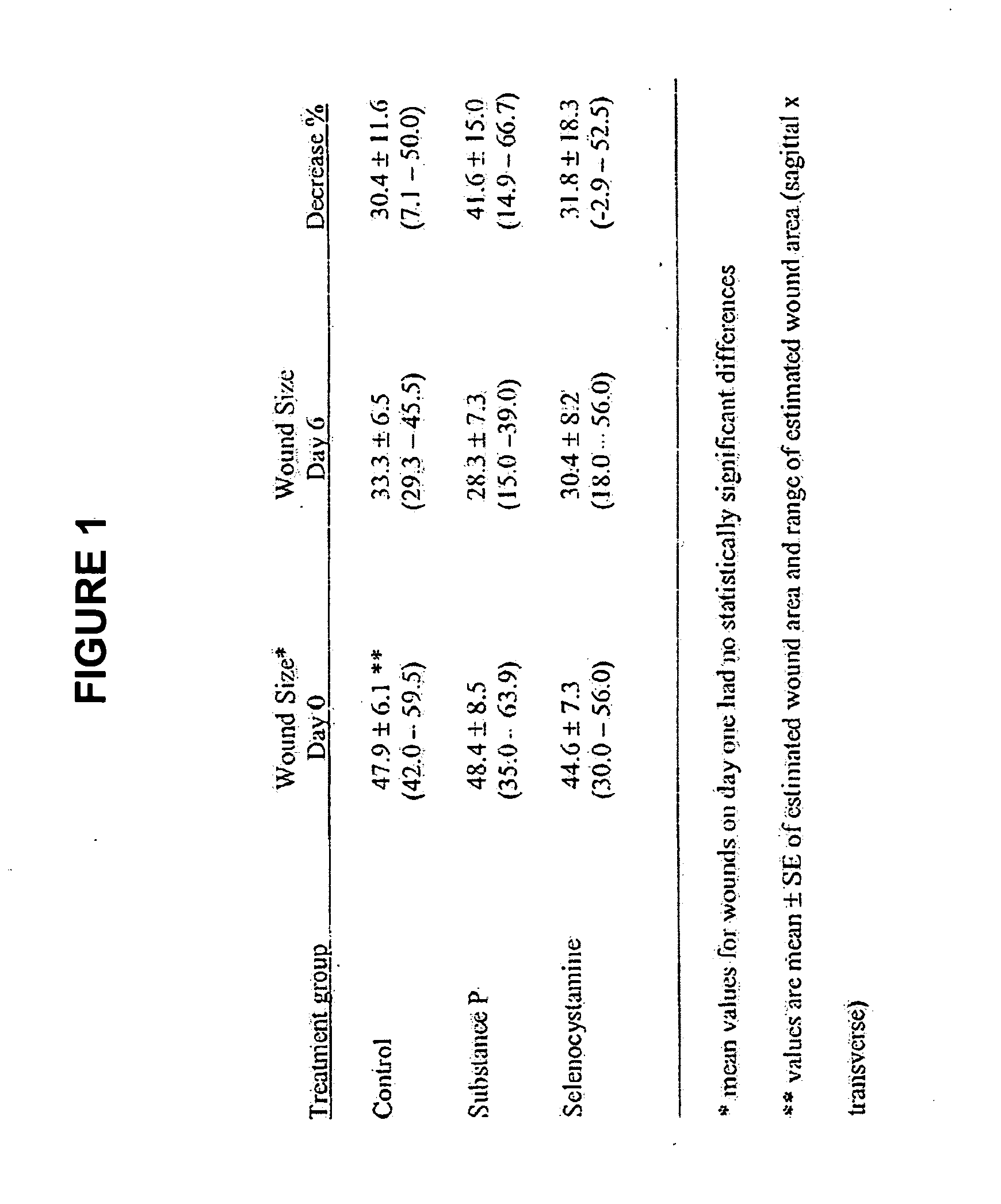
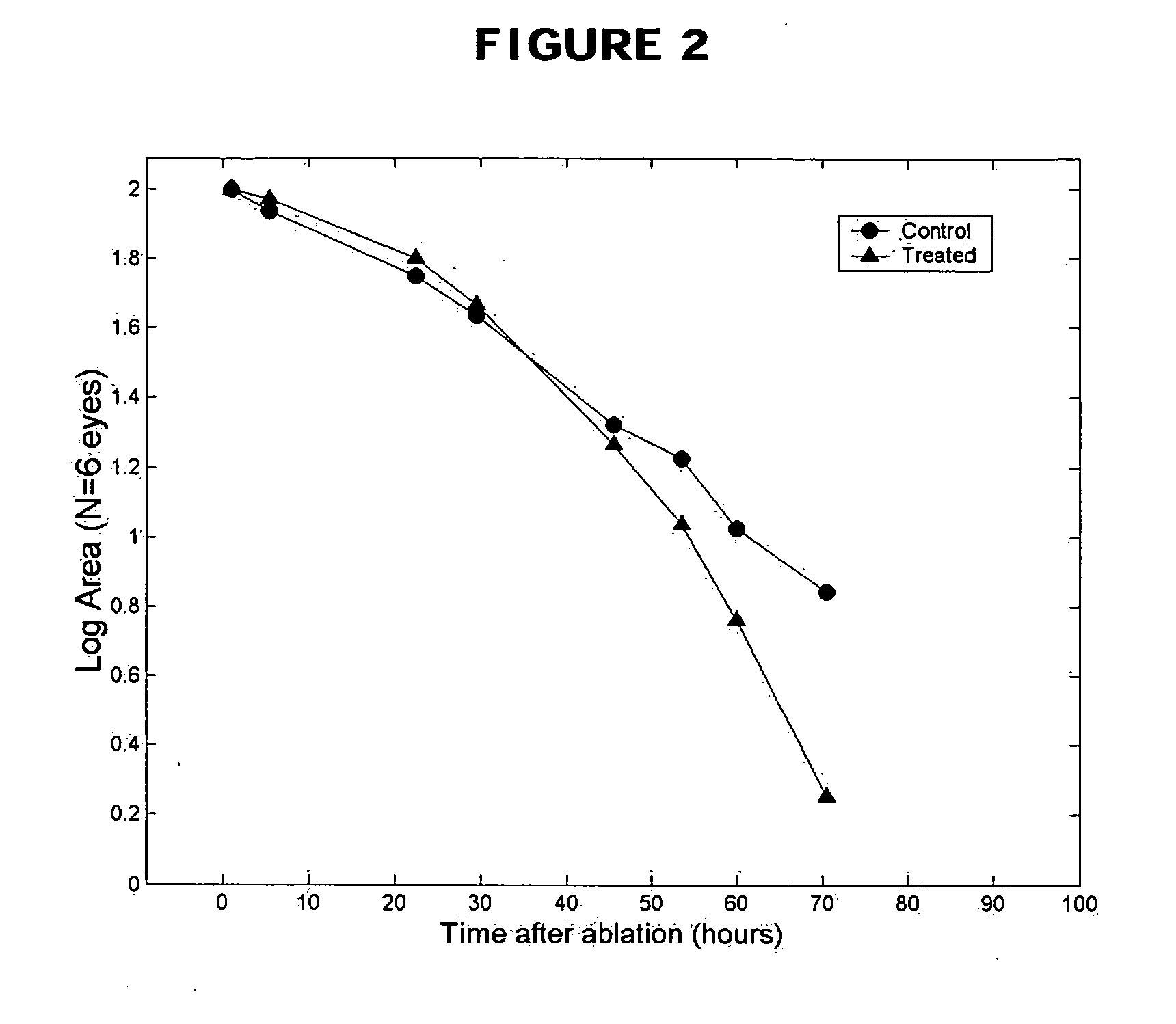
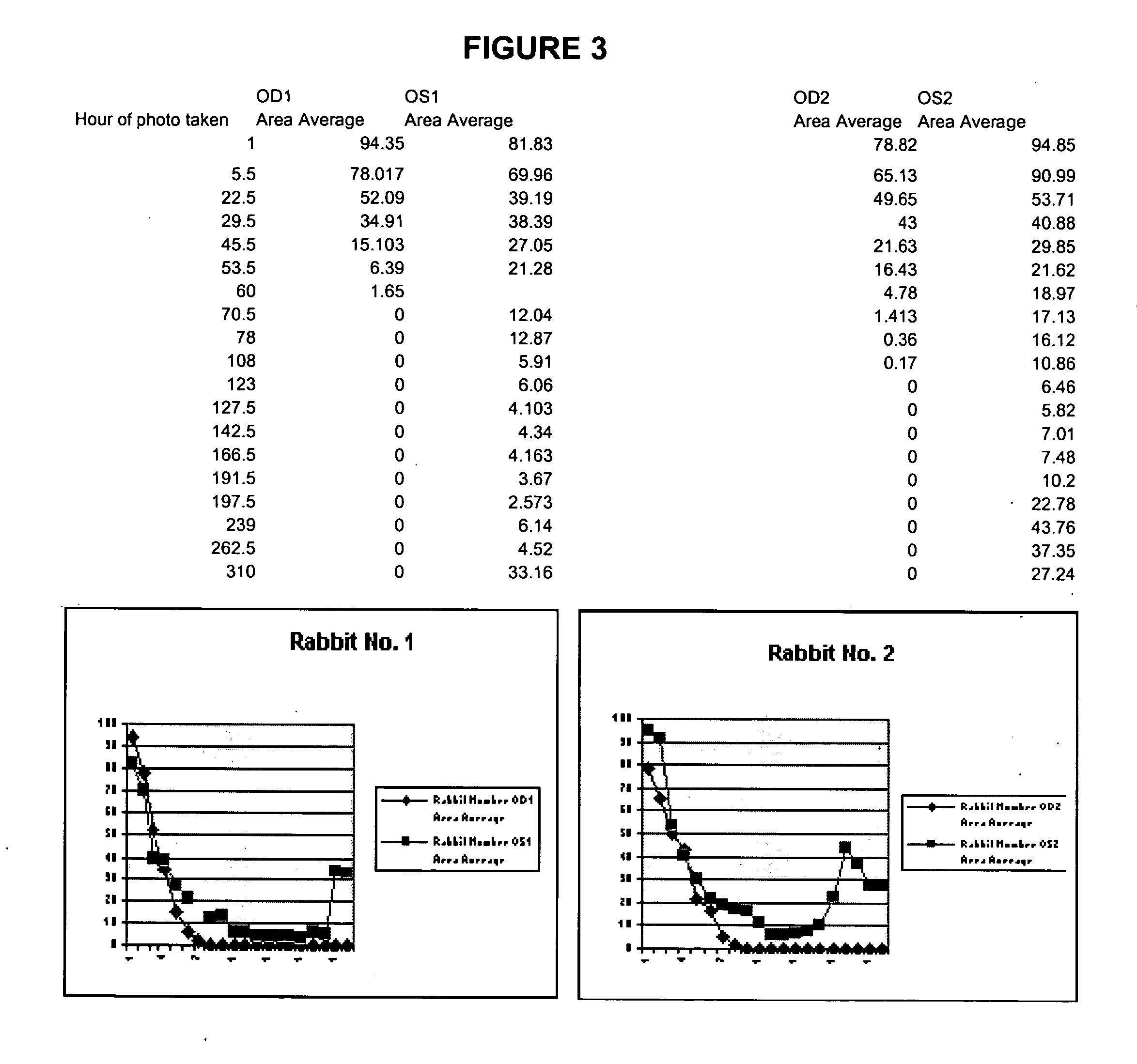
Methods and compositions using Substance P to promote wound healing
InactiveUS20070154448A1Promote healingGrowth promoting activityOrganic active ingredientsBiocideSubstance KMammalian tissue
Healing of wounds in mammalian tissue may be enhanced by the application of certain neuropeptides, optionally in combination with known growth promoting hormones. Exemplary neuropeptides include tachykinins, such as Substance P, Substance K, and the like, as well as calcitonin gene-related peptides. The compositions may further include a polymeric delivery carrier and are utilized by applying to the site of the wound. Wounds may be vascular or avascular wounds. The compositions promote elaboration of cellular matrices and development of cellular attachment mechanisms in addition to stimulating cellular proliferation.
Owner:AUXANO BIOLOGICS
Antagonist antibodies directed against calcitonin gene-related peptide and methods using same
ActiveUS8007794B2Reduce developmentDelay progressOrganic active ingredientsNervous disorderVasomotor symptomCalcitonin gene-related peptide
The invention features methods for preventing or treating CGRP associated disorders such as vasomotor symptoms, including headaches (e.g., migraine, cluster headache, and tension headache) and hot flushes, by administering an anti-CGRP antagonist antibody. Antagonist antibody G1 and antibodies derived from G1 directed to CGRP are also described.
Owner:TEVA PHARMACEUTICALS INTERNATIONAL GMBH
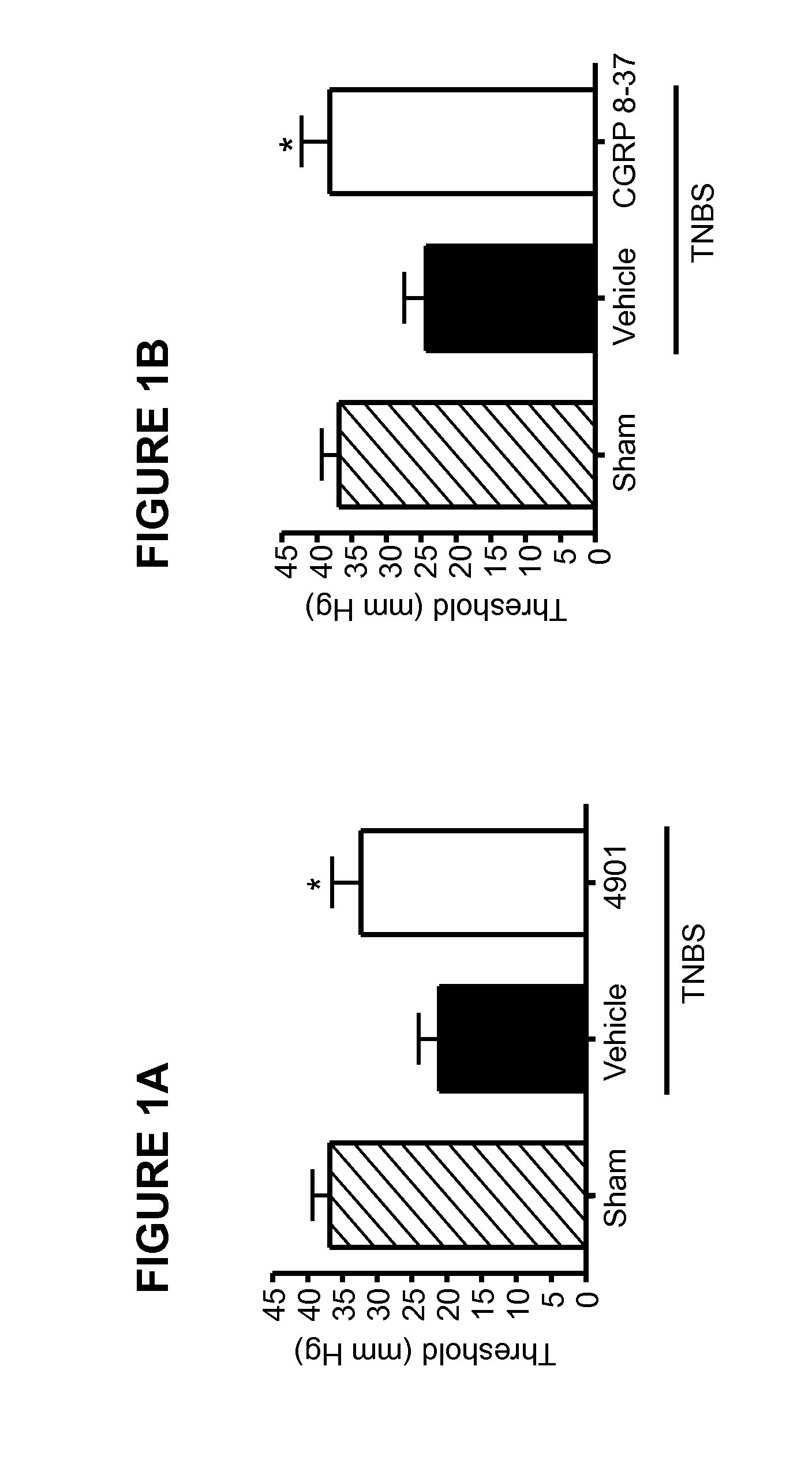
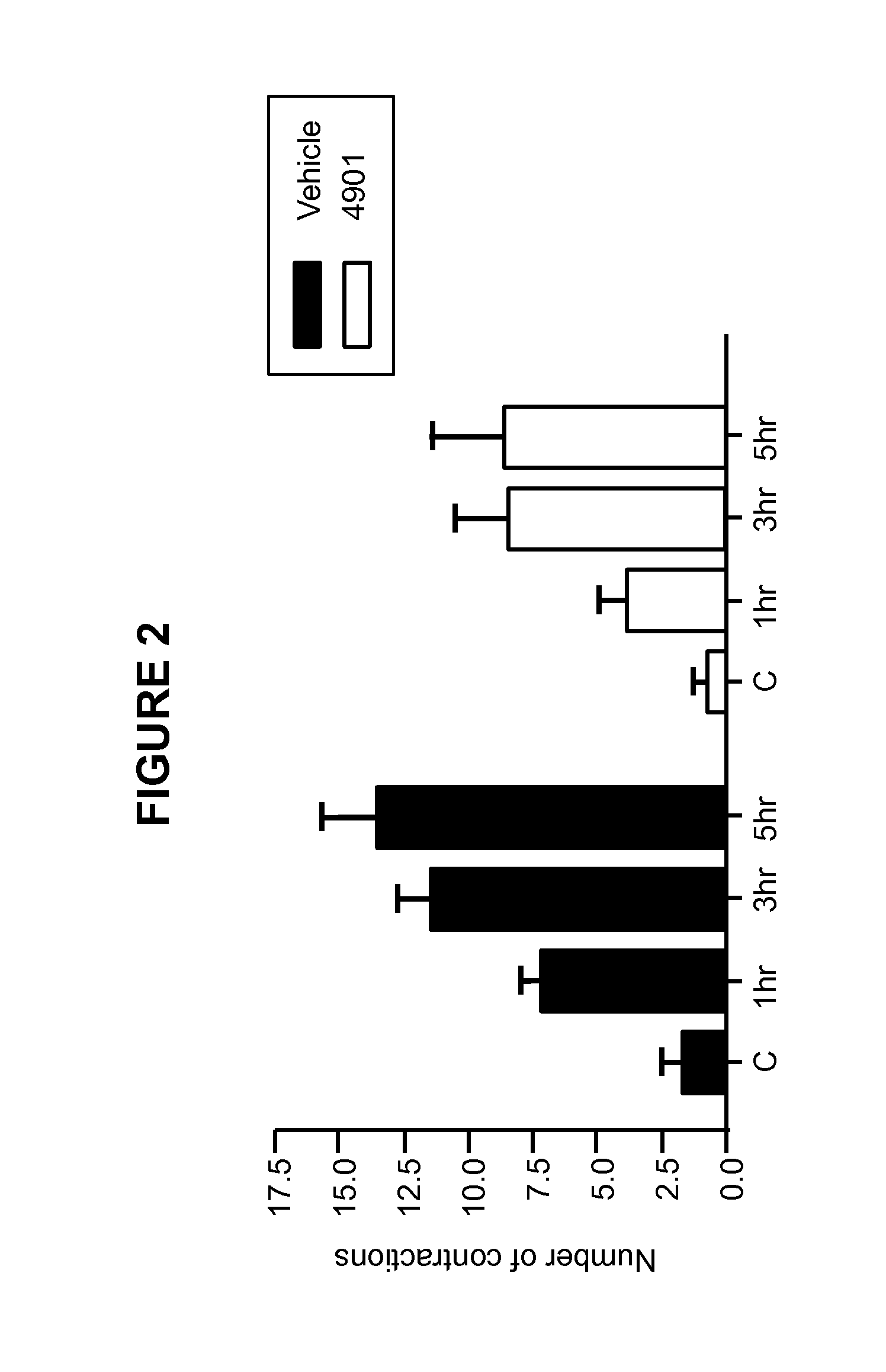
Methods for treating visceral pain by administering antagonist antibodies directed against calcitonin gene-related peptide
The invention features methods for preventing or treating visceral pain, including pain associated with functional bowel disorder, inflammatory bowel disease and interstitial cystitis, by administering an anti-CGRP antagonist antibody.
Owner:TEVA PHARMACEUTICALS INTERNATIONAL GMBH
Methods for treating visceral pain by administering antagonist antibodies directed against calcitonin gene-related peptide
The invention features methods for preventing or treating visceral pain, including pain associated with functional bowel disorder, inflammatory bowel disease and interstitial cystitis, by administering an anti-CGRP antagonist antibody.
Owner:TEVA PHARMACEUTICALS INTERNATIONAL GMBH
Method of Improving Treatments in Rheumatic and Arthritic Diseases
Improved treatments of joint diseases, such as, e.g. osteoarthritis and rheumatoid arthritis, and pain, wherein a strontium-containing compound is administered alone or in combination with one or more second therapeutically and / or prophylactically active substances, selected from the group consisting of bisphosphonates, glucosamine, pallitative agents, analgesic agents, disease modifying anti-rheumatic compounds (DMARDs), selective estrogen receptor modulators (SERMs), aromatase inhibitors, non-steroidal anti-inflammatory agents (NSAIDs), COX-2 inhibitors, COX-3 inhibitors, opioids, inhibitors / antagonists of IL-1, inhibitors / antagonists of TNF-alpha, inhibitors of matrix metallo-proteinases (MMPs), cathepsin K inhibitors, inhibitors / antagonists of RANK-ligand, statins, glucocorticoids, chondroitin sulphate, NMDA receptor antagonists, inhibitors of interleukin-I converting enzyme, Calcitonin gene related peptide antagonists, glycine antagonists, vanilloid receptor antagonists, inhibitors of inducible nitric oxide synthetase (iNOS), N-acetylcholine receptor agonists, neurokinin antagonists, neuroleptic agents, PAR2 receptor antagonists and anabolic growth factors acting on joint tissue components. Pharmaceutical compositions comprising a strontium-containing compound and a second therapeutically and / or prophylactically active substance as defined above.
Owner:OSTEOLOGIX AS
Antagonist antibodies directed against calcitonin gene-related peptide and methods using same
ActiveUS20150266948A1Reduce developmentDelay progressNervous disorderImmunoglobulins against animals/humansVasomotor symptomCluster headache
The invention features methods for preventing or treating CGRP associated disorders such as vasomotor symptoms and / or headaches (e.g., migraine, cluster headache, and tension headache) by administering an anti-CGRP antagonist antibody. Compositions for use in the disclosed methods are also provided. Antagonist antibody G1 and antibodies derived from G1 directed to CGRP are also described.
Owner:TEVA PHARMACEUTICALS INTERNATIONAL GMBH
Antagonist antibodies directed against calcitonin gene-related peptide and methods using same
ActiveUS20150322142A1Reduce developmentDelay progressNervous disorderImmunoglobulins against animals/humansVasomotor symptomCluster headache
The invention features methods for preventing or treating CGRP associated disorders such as vasomotor symptoms and / or headaches (e.g., migraine, cluster headache, and tension headache) by administering an anti-CGRP antagonist antibody. Compositions for use in the disclosed methods are also provided. Antagonist antibody G1 and antibodies derived from G1 directed to CGRP are also described.
Owner:TEVA PHARMACEUTICALS INTERNATIONAL GMBH
Antagonist antibodies directed against calcitonin gene-related peptide and methods using same
ActiveUS20120009192A1Reduce developmentDelay progressOrganic active ingredientsFungiVasomotor symptomCalcitonin
The invention features methods for preventing or treating CGRP associated disorders such as vasomotor symptoms, including headaches (e.g., migraine, cluster headache, and tension headache) and hot flushes, by administering an anti-CGRP antagonist antibody. Antagonist antibody G1 and antibodies derived from G1 directed to CGRP are also described.
Owner:TEVA PHARMACEUTICALS INTERNATIONAL GMBH
Use of neuropeptides for ligament healing
InactiveUS7776815B2High strengthDistinct utilityTachykinin ingredientsImmunoglobulinsDiseaseThyrotropin-releasing hormone
Disclosed are a method and a corresponding pharmaceutical composition for treating damaged ligaments. Neurogenic compounds in general and neuropeptides in particular have been found to be highly effective in stimulated repair of ligaments damaged due to traumatic injury, ligament disease, and disuse. Preferred active ingredients for use in the method and corresponding pharmaceutical composition include calcitonin gene-related peptide (CGRP), cholecystokinin (CCK), dynorphin, enkephalin, galanin, neuropeptide Y (NPY), neurotensin, somatostatin, substance P (SP), thyrotropin-releasing hormone (TRH), vasoactive intestinal peptide (VIP).
Owner:WISCONSIN ALUMNI RES FOUND
Neuropeptide release assay for sodium channels
ActiveUS8486647B2High activityReduced activityCell receptors/surface-antigens/surface-determinantsSugar derivativesDiseaseBiological activation
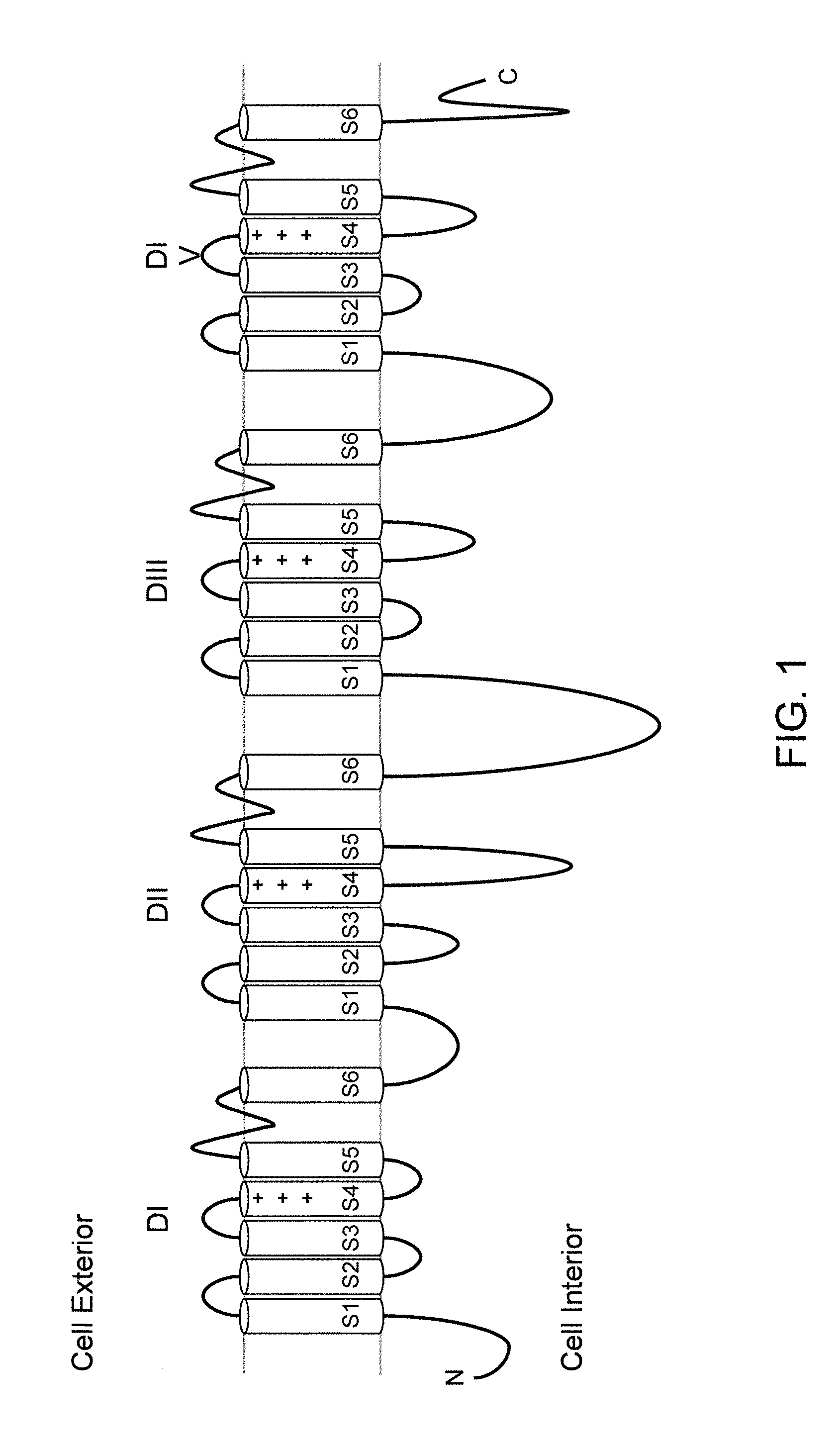
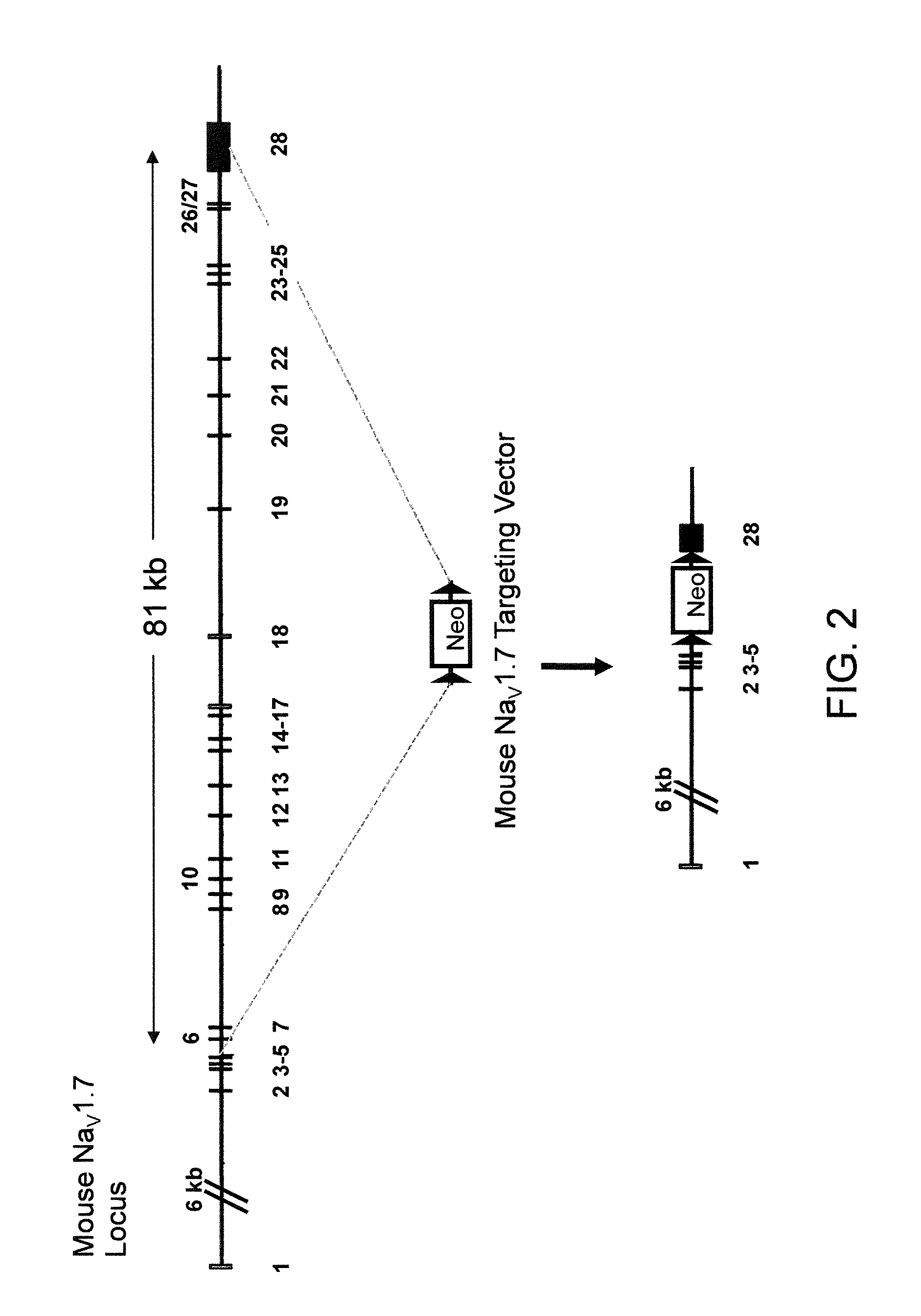
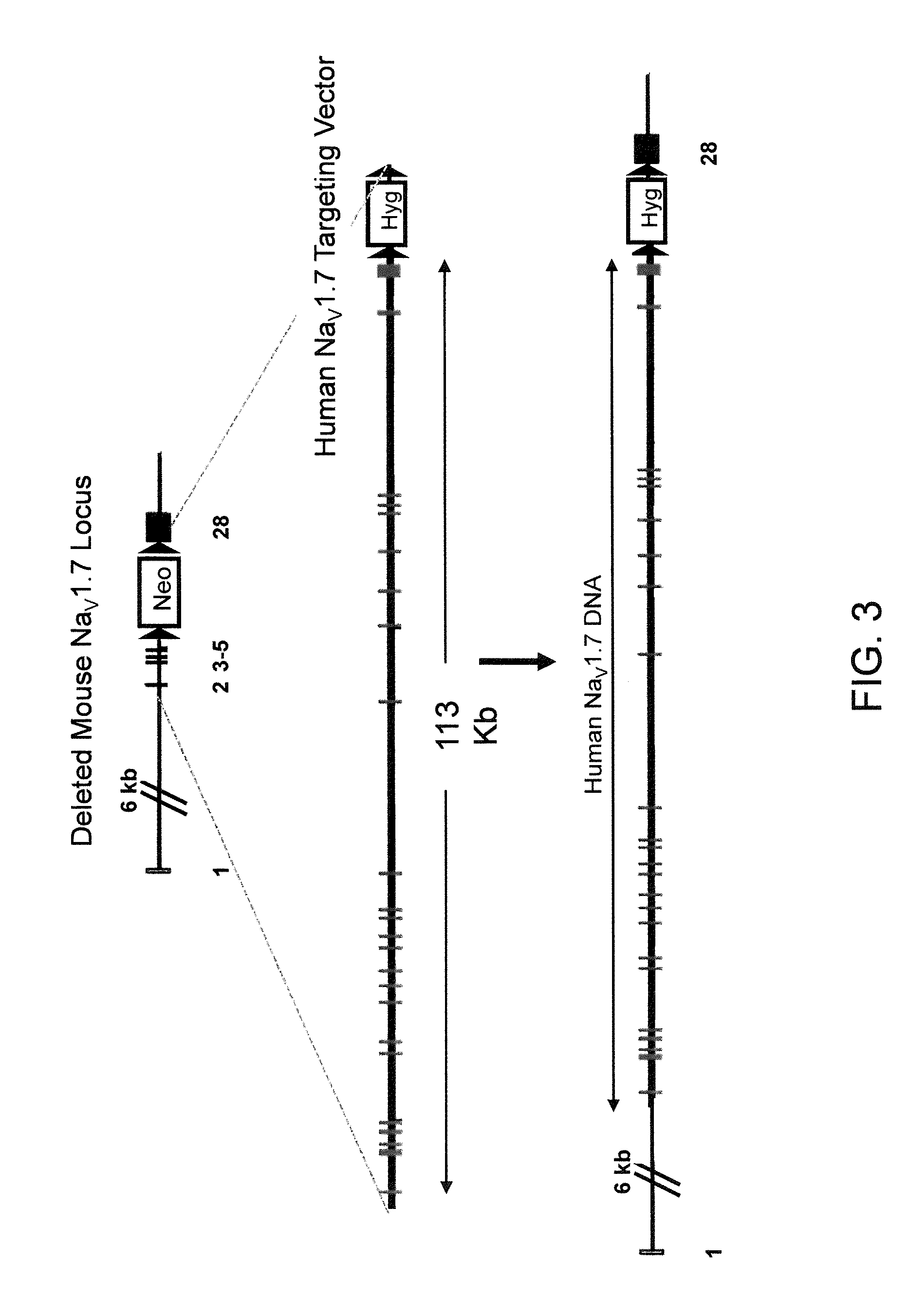
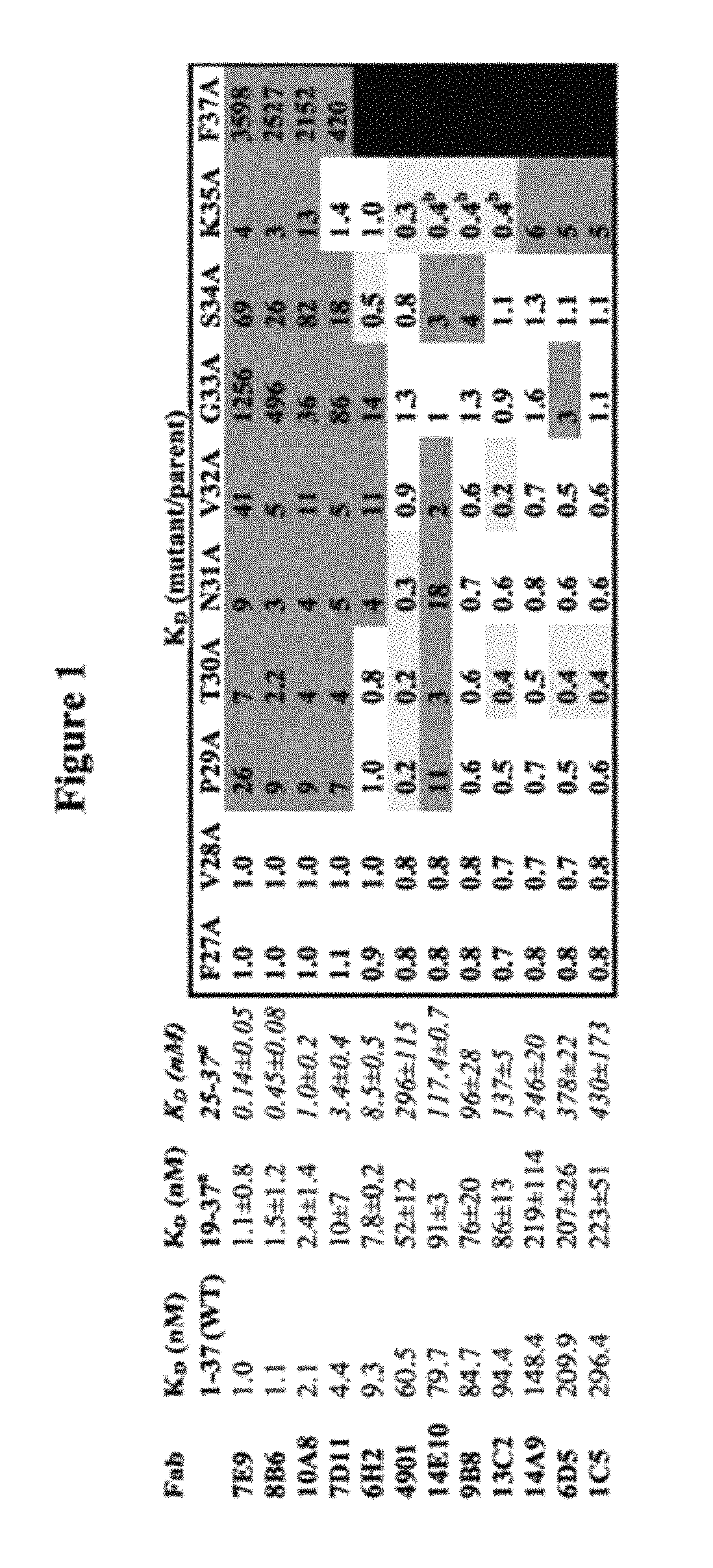
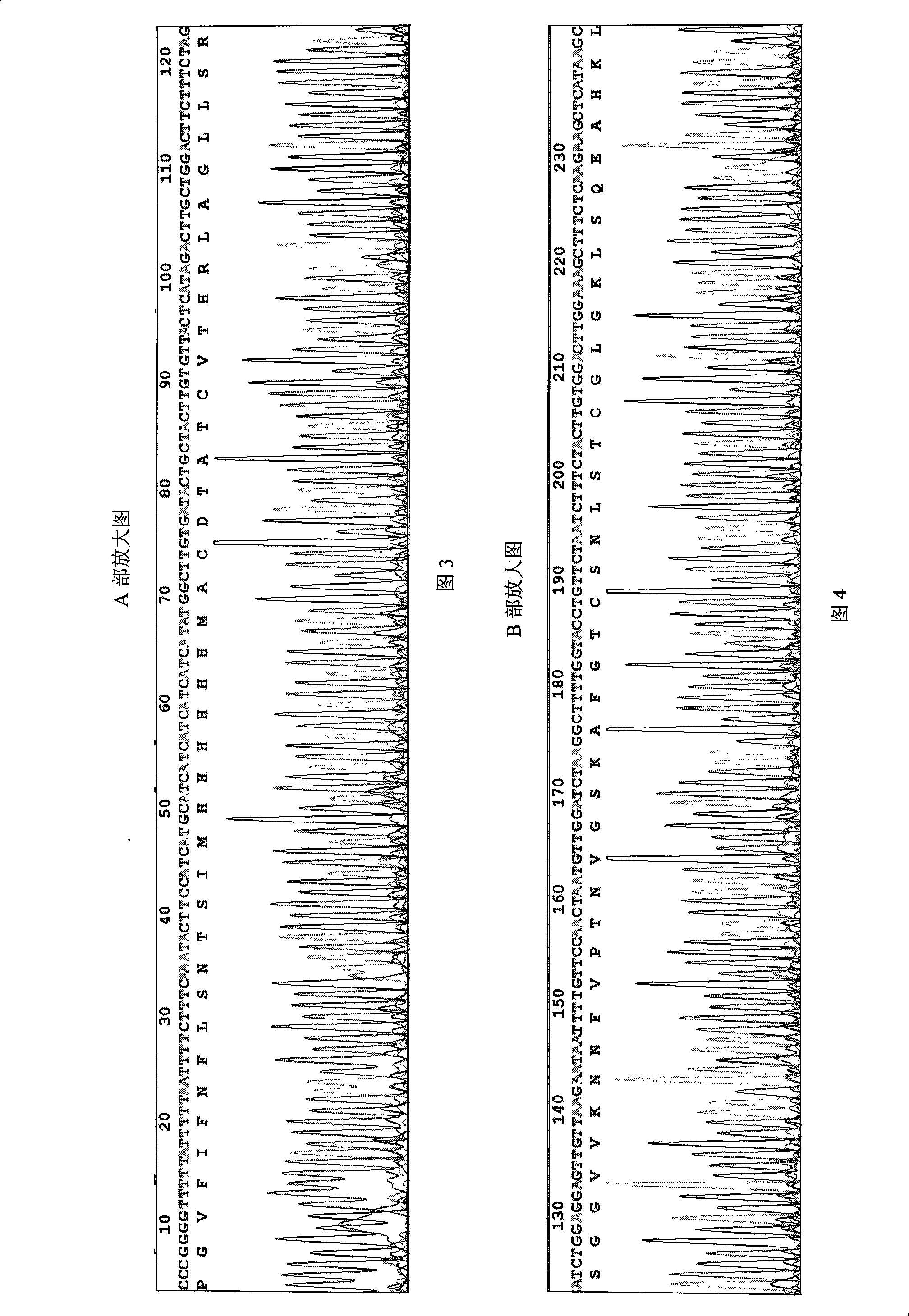
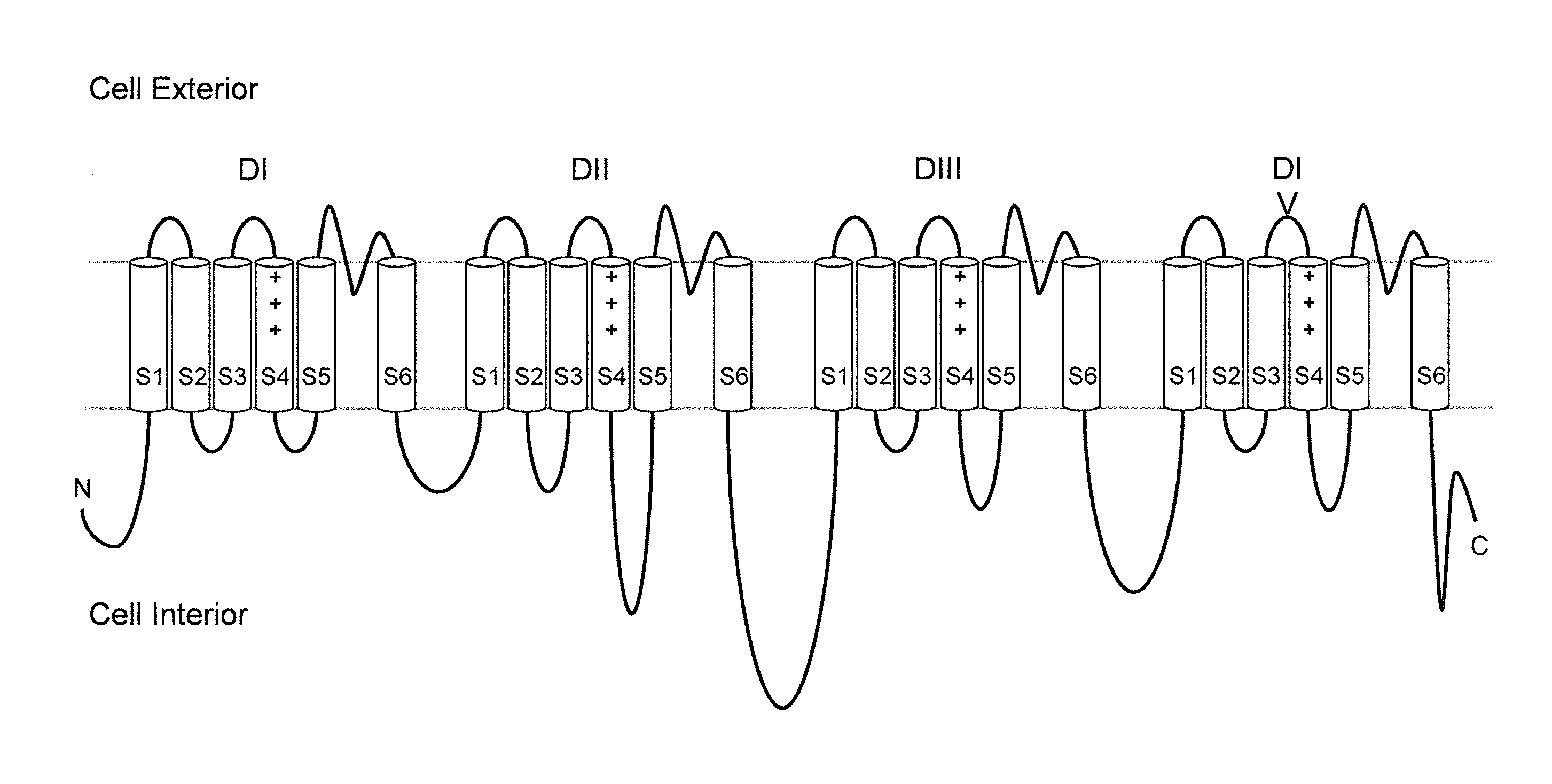
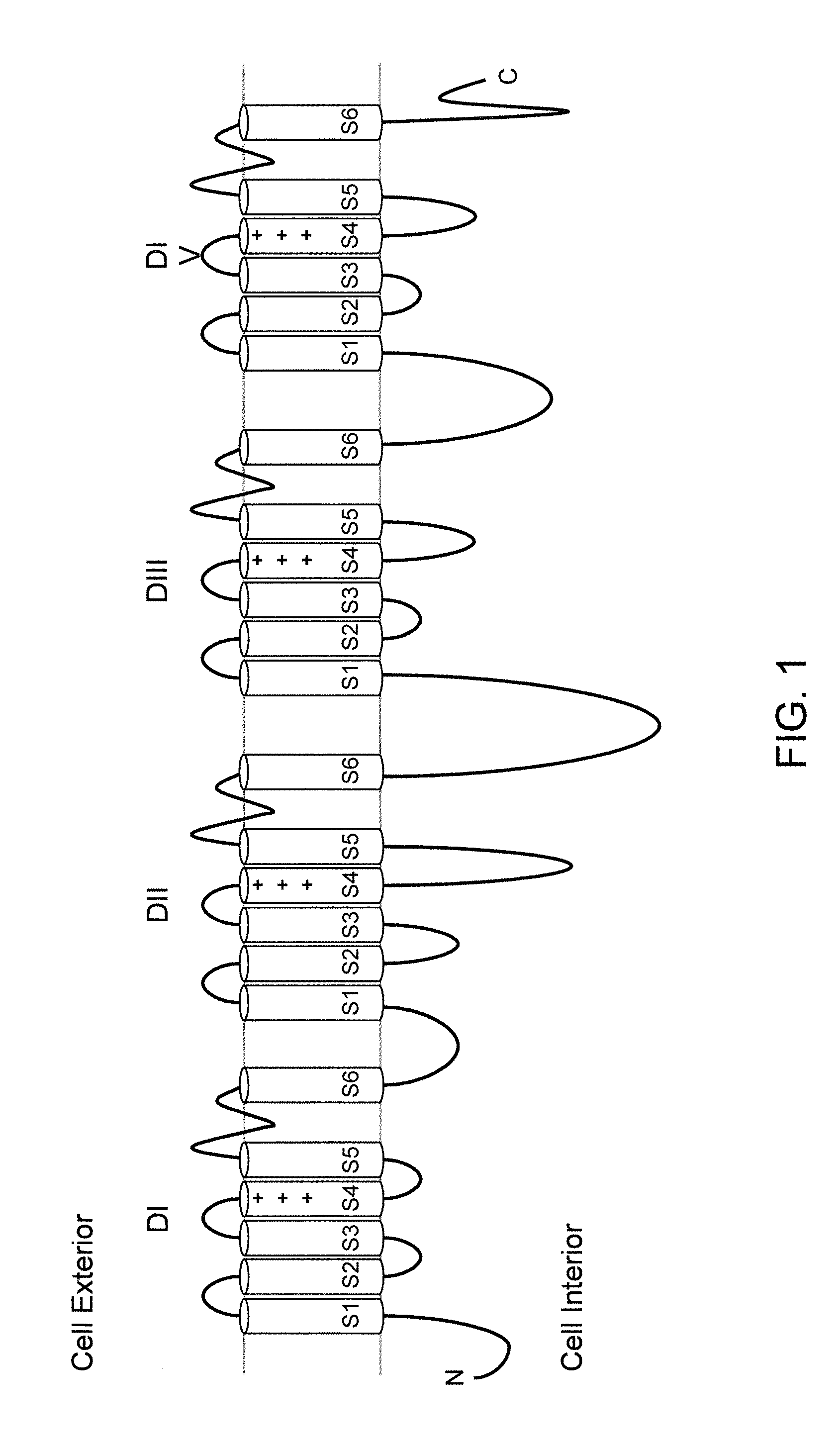
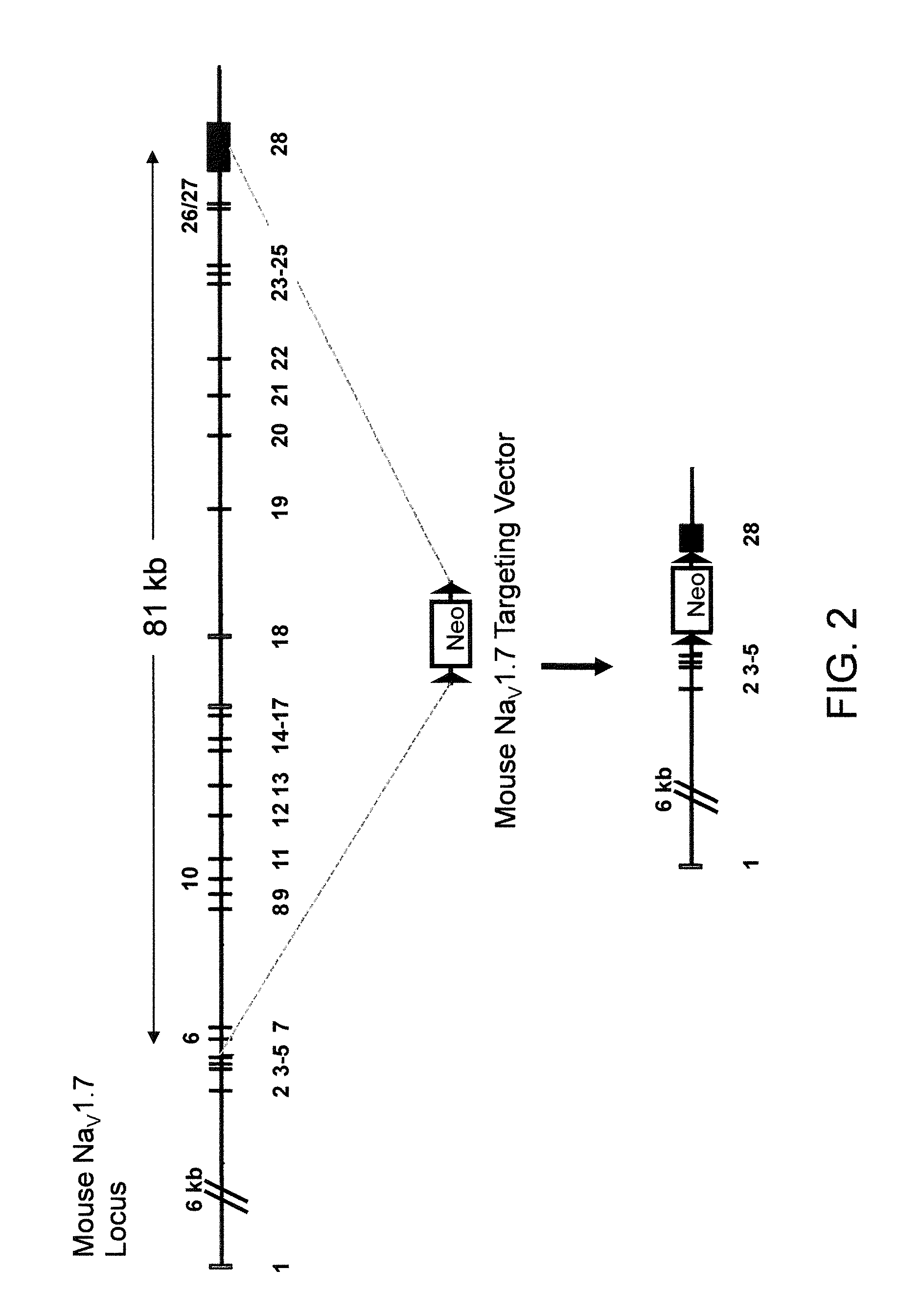
Methods and compositions for using genetically modified non-human animals are provided, wherein the genetic modification comprises a humanization of the one or more extracellular pore loops of a NaV1.7 channel protein or a complete humanization of an endogenous NaV1.7 gene. Methods for using isolated DRG cultures from genetically modified non-human animals are also provided, wherein the isolated DRG express a human or chimeric NaV1.7 protein on the surface, in particular measuring primary nociceptive activation through the release of calcitonin gene-related peptide (CGRP) in isolated DRG in vitro, and wherein the isolated DRG cultures are capable of generating action potentials and communicating through an excitable signal via the expressed human or chimeric NaV1.7 protein the cell surface. In vivo and in vitro methods for characterizing NaV1.7-specific antagonists and evaluation of corresponding therapeutic potential for NaV1.7-mediated disease are also provided.
Owner:REGENERON PHARM INC
Antagonist antibodies directed against calcitonin gene-related peptide and methods using same
ActiveUS9896502B2Reduce developmentDelay progressNervous disorderImmunoglobulins against animals/humansVasomotor symptomCluster headache
The invention features methods for preventing or treating CGRP associated disorders such as vasomotor symptoms and / or headaches (e.g., migraine, cluster headache, and tension headache) by administering an anti-CGRP antagonist antibody. Compositions for use in the disclosed methods are also provided. Antagonist antibody G1 and antibodies derived from G1 directed to CGRP are also described.
Owner:TEVA PHARMACEUTICALS INTERNATIONAL GMBH
Calcitonin-gene-related peptide and trout calcitonin amalgamation polypeptide transgenic sequence, and transgenic engineering bacterial strain
InactiveCN101319223AEffective ELISAInexpensive and effective in field cultivationBacteriaMicroorganism based processesRecombinant salmon calcitoninEscherichia coli
The invention provides a fusion polypeptide transgene sequence for calcitonin-gene-related peptide and salmon calcitonin and a transgene engineering strain thereof, and relates to a fusion polypeptide transgene sequence and a transgene engineering strain. The invention solves the problem that: the prior calcitonin-gene-related peptide and salmon calcitonin fusion polypeptide are not suitable for industrialized production with microbes and animals as reactors. The calcitonin-gene-related peptide and salmon calcitonin fusion polypeptide transgene sequence is shown as SEQ ID NO.1. The calcitonin-gene-related peptide and salmon calcitonin fusion polypeptide transgene engineering strain is an agrobacterium containing fusion gene plasmids, wherein the fusion genes in the plasmids are shown as SEQ ID NO.1. The calcitonin-gene-related peptide and salmon calcitonin fusion polypeptide transgene engineering strain can be used for mediating a plant with the cost of the engineering strain being 1 / 10 to 1 / 50 of that of bacillus coli.
Owner:HEILONGJIANG UNIV
Neuropeptide Release Assay For Sodium Channels
Methods and compositions for using genetically modified non-human animals are provided, wherein the genetic modification comprises a humanization of the one or more extracellular pore loops of a NaV1.7 channel protein or a complete humanization of an endogenous NaV1.7 gene. Methods for using isolated DRG cultures from genetically modified non-human animals are also provided, wherein the isolated DRG express a human or chimeric NaV1.7 protein on the surface, in particular measuring primary nociceptive activation through the release of calcitonin gene-related peptide (CGRP) in isolated DRG in vitro, and wherein the isolated DRG cultures are capable of generating action potentials and communicating through an excitable signal via the expressed human or chimeric NaV1.7 protein the cell surface. In vivo and in vitro methods for characterizing NaV1.7-specific antagonists and evaluation of corresponding therapeutic potential for NaV1.7-mediated disease are also provided.
Owner:REGENERON PHARM INC
Applications of Chinese medicinal composition in preparing medicament for improving function of vascular endothelium
InactiveCN101322792AReduce plasma endothelinLower plasma endothelin (ET)Cardiovascular disorderPlant ingredientsVascular endotheliumNitric oxide
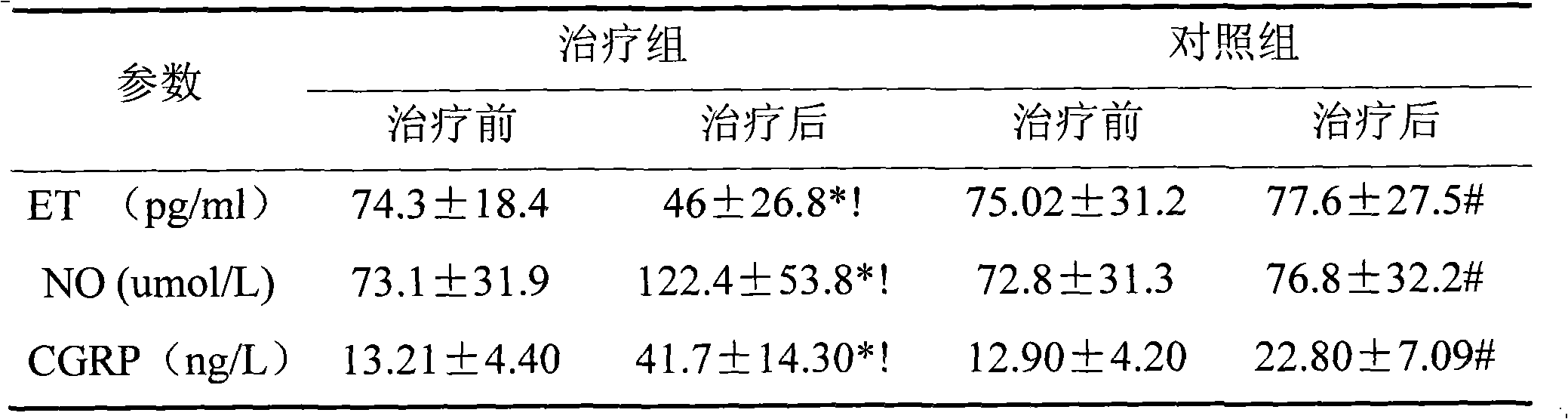
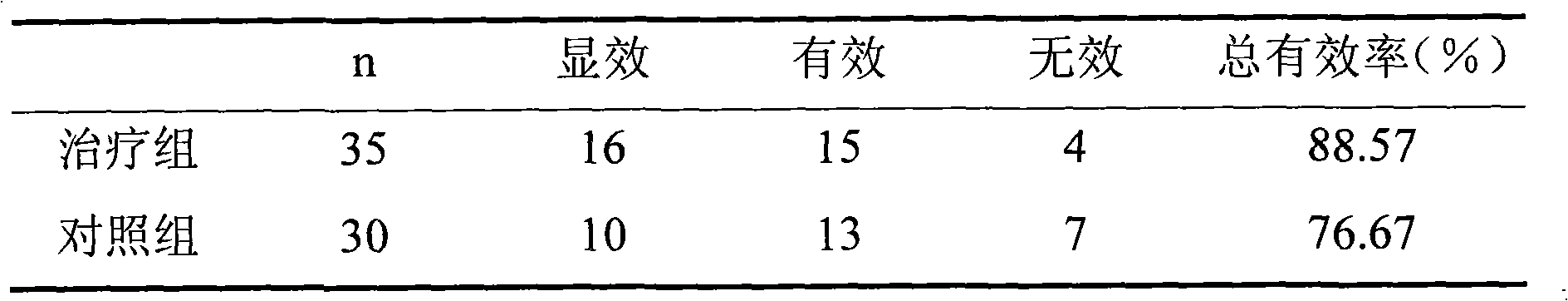
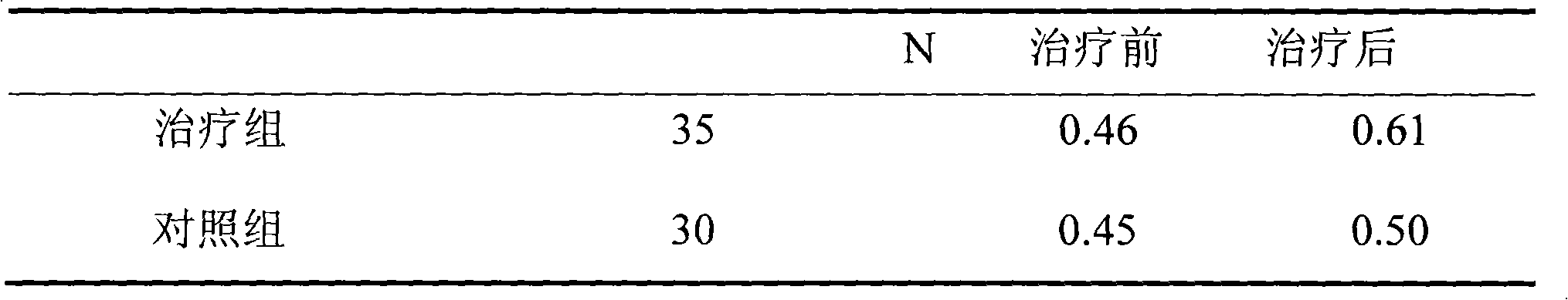
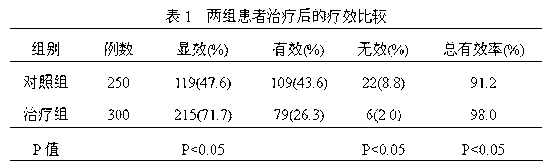
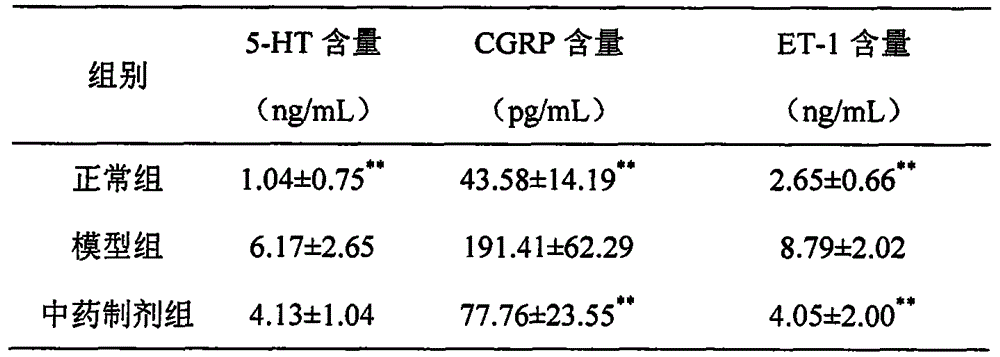
The invention discloses an application of a traditional Chinese medicine composition in preparation of a medicine for improving the endothelium function of blood vessels. The traditional Chinese medicine composition of the invention can effectively decrease plasma endothelin (ET) of patients with heart failure, improve the level of nitric oxide (NO) and calcitonin gene-related peptide (CGRP); the traditional Chinese medicine composition of the invention can effectively improve the endothlelium function of blood vessels of patients with chronic heart failure, and the symptoms of heart failure and degree thereof are also reduced with the improvement of the endothelium function of blood vessels.
Owner:HEBEI YILING MEDICINE INST
Calcitonin-gene-related peptide and trout calcitonin amalgamation polypeptide
The invention provides calcitonin gene-related peptide and salmon calcitonin fusion polypeptide, relating to fusion polypeptide. In order to generate a product containing both calcitonin and the calcitonin gene-related peptide, the invention provides the calcitonin gene-related peptide and the salmon calcitonin fusion polypeptide. The amino acid sequence of the calcitonin gene-related peptide and the salmon calcitonin fusion polypeptide is shown in SEQ ID No:1; moreover, C-terminal amidation is carried out to the calcitonin gene-related peptide and the salmon calcitonin fusion polypeptide. Experiments verify that the calcitonin gene-related peptide and the salmon calcitonin fusion polypeptide preserve respective activities of the calcitonin gene-related peptide and salmon calcitonin.
Owner:HEILONGJIANG UNIV
Peptide antagonists of the calcitonin cgrp family of peptide hormones and their use
The embodiments provide a modified calcitonin gene-related peptide antagonist including an N-terminal fragment of modified calcitonin gene-related peptide or related protein family member where at least two residues of the N-terminal fragment are cysteine (Cys) and at least one amino acid comprises a non-threonine substitution of a threonine (Thr) residue; a central core where the central core comprises an oc-helix; and a C-terminal fragment of modified calcitonin gene-related peptide or related protein family member comprising a C-terminal amide and where at least one amino acid of the C-terminal fragment is phenylalanine (Phe), proline (Pro), tyrosine (Tyr) or hydroxyproline (Hyp) or pharmaceutically acceptable salt thereof, as well as compositions, including pharmaceutical compositions, comprising a subject peptide. The embodiments further provide treatment methods, including methods of treating a migraine, the methods generally involving administering to an individual in need thereof an effective amount of a subject peptide or composition.
Owner:SOARES CHRISTOPHER J
Medicine for treating protrusion of lumbar intervertebral disc
ActiveCN103316209ARaise the pain threshold of mechanical stimulationAnthropod material medical ingredientsSkeletal disorderCentipedeMyrrh
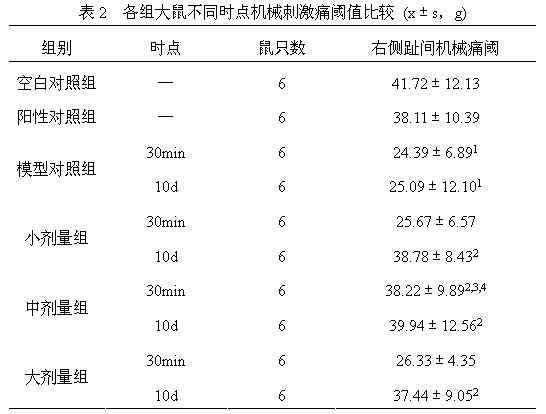
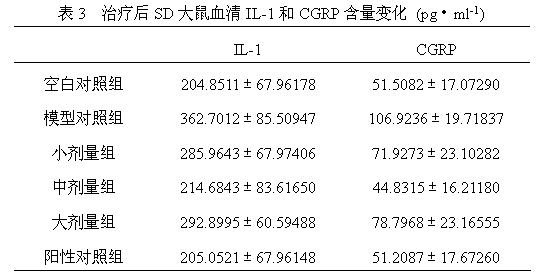
The invention discloses a medicine for treating protrusion of lumbar intervertebral disc. The raw material medicines including unprocessed radix astragali, centipede, scorpio, pangolin scales, eucommia ulmoides, dipsacus root, honeysuckle, zaocys dhumnade, Chinese angelica, radix paeoniae rubra, frankincense, myrrh, rhizoma corydalis, fructus psoraleae, rhizoma drynariae, radix clematidis, uncaria rhynchophylla, caulis sinomenii, kadsura pepper stem, caulis spatholobi, radix asparagi and radix ophiopogonis are ground into powder. The medicine disclosed by the invention has the functions of tonifying qi, invigorating kidney, dredging muscles, strengthening bone, activating blood, resolving stasis and dredging collaterals and relieving pain, realizes both dredging and replenishment and treatment on both symptoms and root causes when used for treating protrusion of lumbar intervertebral disc. According to the observation of the clinical curative effect on 300 patients, the total effective rate is 98%; and animal experiments indicate that the medicine disclosed by the invention obviously improves the IL-1 and CGRP (Calcitonin Gene-Related Peptide) in the serum of rat of a model for protrusion of lumbar intervertebral disc, and can remarkably improve the threshold of mechanical stimulus pain of the rat of the model for protrusion of lumbar intervertebral disc.
Owner:山西省针灸研究所
Use of neuropeptides for ligament healing
InactiveUS20060030942A1Promote ligamentPromote tendon healingTachykinin ingredientsImmunoglobulinsLigament healingThyrotropin-releasing hormone
Disclosed are a method and a corresponding pharmaceutical composition for treating damaged ligaments. Neurogenic compounds in general and neuropeptides in particular have been found to be highly effective in stimulated repair of ligaments damaged due to traumatic injury, ligament disease, and disuse. Preferred active ingredients for use in the method and corresponding pharmaceutical composition include calcitonin gene-related peptide (CGRP), cholecystokinin (CCK), dynorphin, enkephalin, galanin, neuropeptide Y (NPY), neurotensin, somatostatin, substance P (SP), thyrotropin-releasing hormone (TRH), vasoactive intestinal peptide (VIP).
Owner:WISCONSIN ALUMNI RES FOUND
Peptide antagonists of the calcitonin CGRP family of peptide hormones and their use
The embodiments provide a modified calcitonin gene-related peptide antagonist including an N-terminal fragment of modified calcitonin gene-related peptide or related protein family member where at least two residues of the N-terminal fragment are cysteine (Cys) and at least one amino acid comprises a non-threonine substitution of a threonine (Thr) residue; a central core where the central core comprises an α-helix; and a C-terminal fragment of modified calcitonin gene-related peptide or related protein family member comprising a C-terminal amide and where at least one amino acid of the C-terminal fragment is phenylalanine (Phe), proline (Pro), tyrosine (Tyr) or hydroxyproline (Hyp) or pharmaceutically acceptable salt thereof, as well as compositions, including pharmaceutical compositions, comprising a subject peptide. The embodiments further provide treatment methods, including methods of treating a migraine, the methods generally involving administering to an individual in need thereof an effective amount of a subject peptide or composition.
Owner:SOARES CHRISTOPHER J
Application of rhizoma chuanxiong total alkaloid to preparation of medicine for treating headache
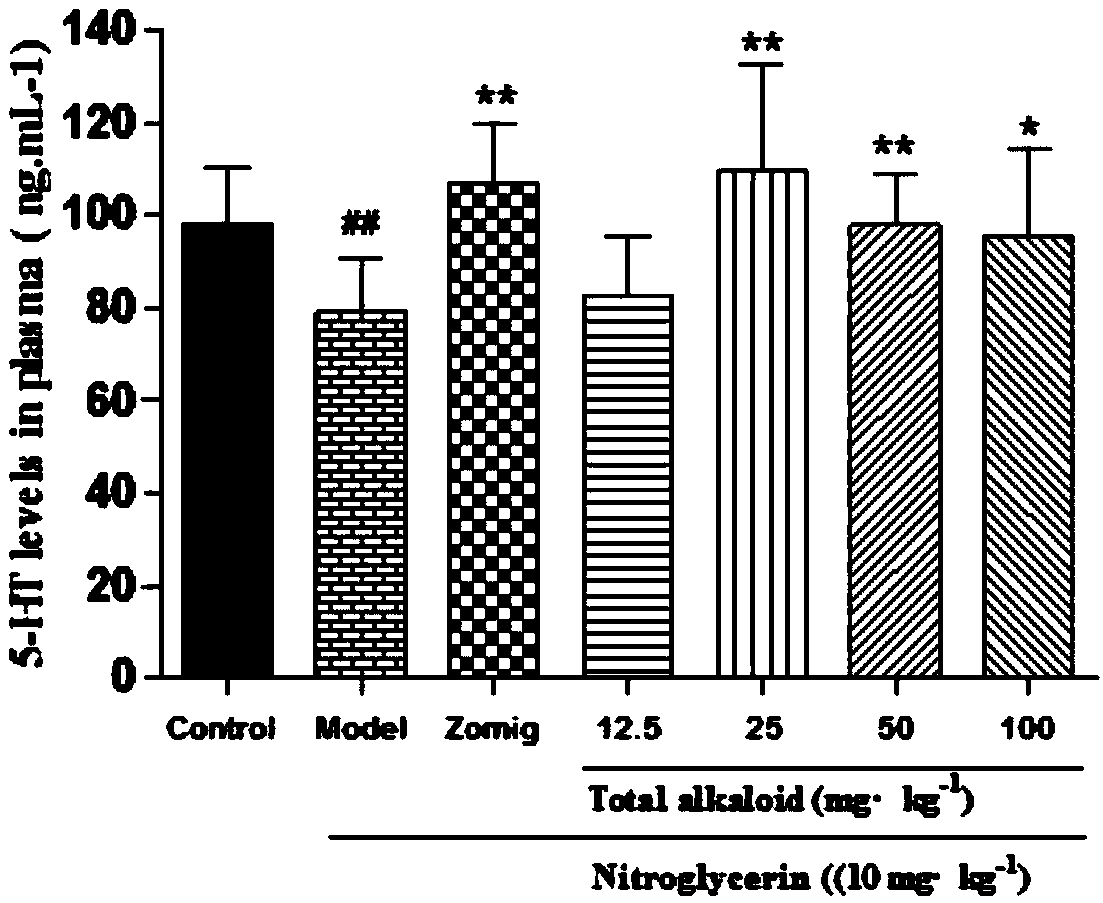
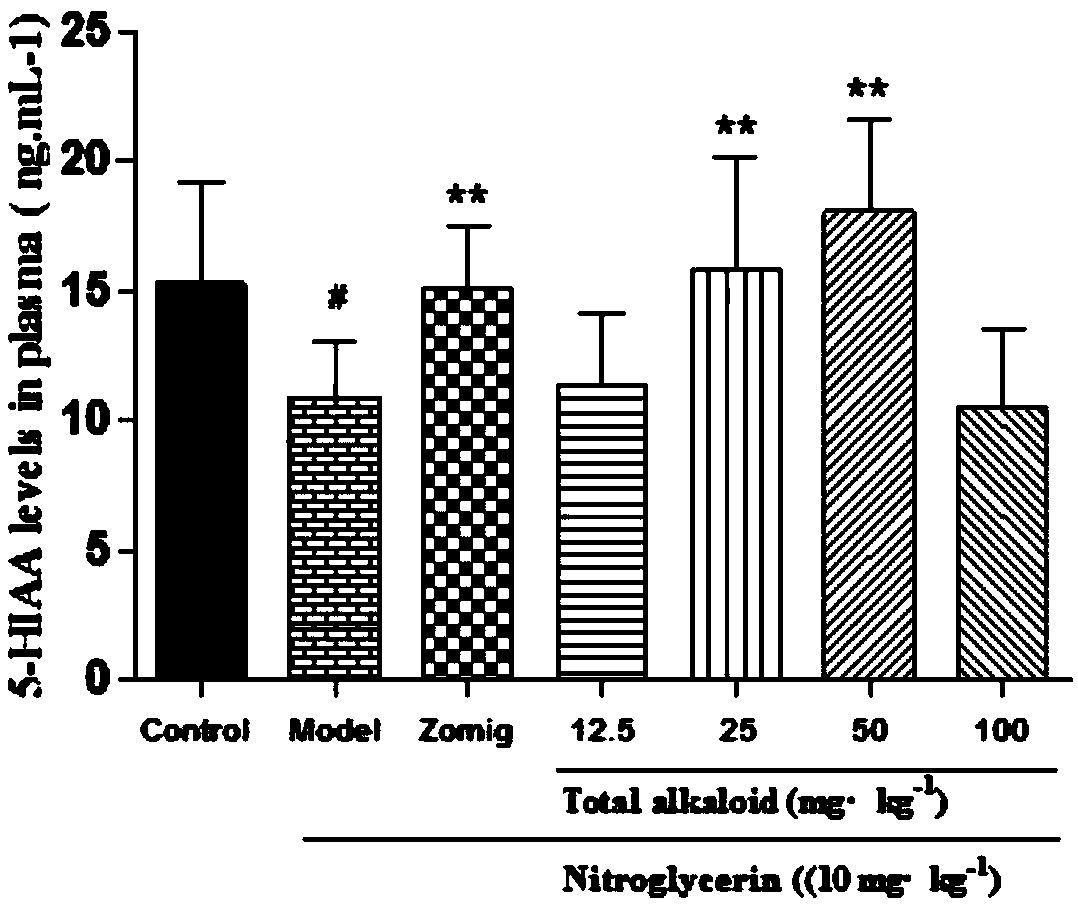
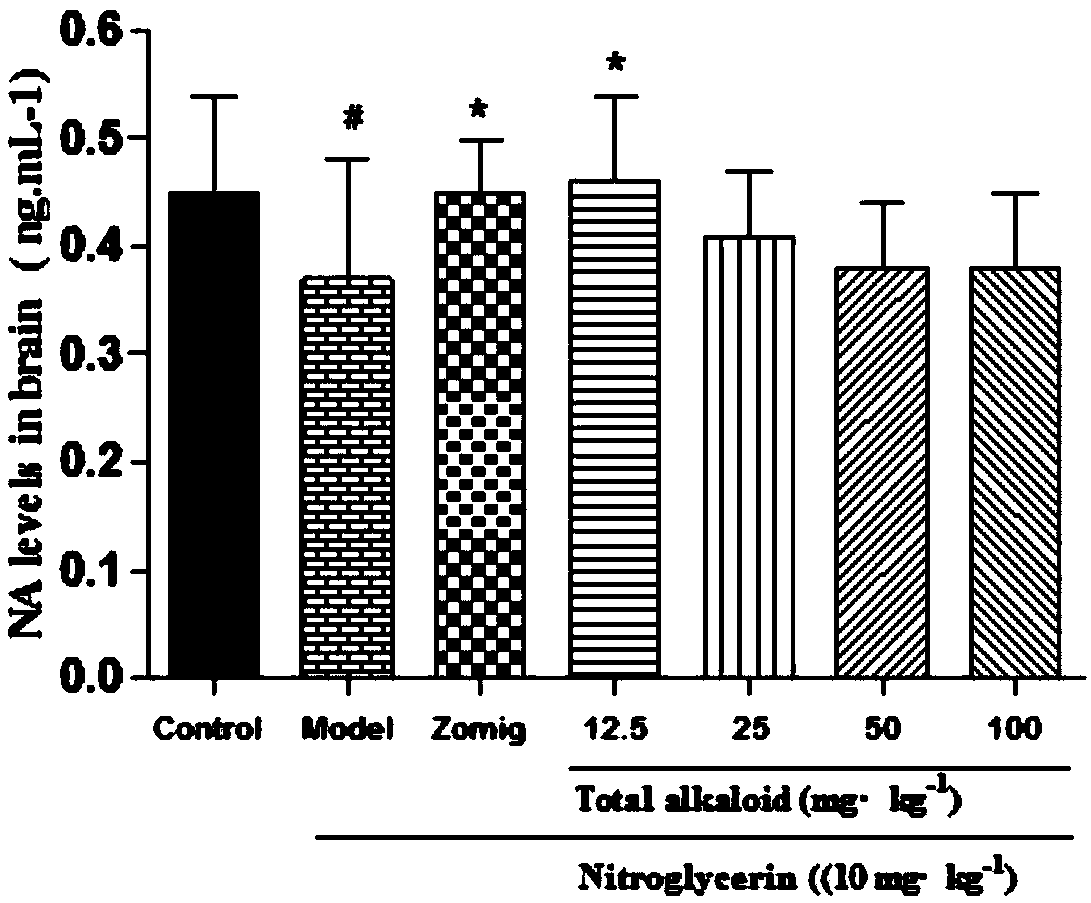
InactiveCN108030794AEffective treatmentLowers CGRP levelsNervous disorderPlant ingredientsAcetic acidHeadaches
The invention provides application of rhizoma chuanxiong total alkaloid to preparation of a medicine for treating headache. According to the application provided by the invention, the rhizoma chuanxiong total alkaloid can be used for improving the content of 5-HT (5-hydroxytryptamine), 5-HIAA (5-Hydroxyindole Acetic Acid), ET (Endothelin), NA (Neuramidinase) and DA (Dopamine) in blood plasma and brain tissues of patients with the headache and reducing a CGRP (Calcitonin Gene-Related Peptide) level in the blood plasma and the brain tissues; the rhizoma chuanxiong total alkaloid can be used foreffectively treating the headache and has a good clinical application prospect.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
Medicine composition for preventing and treating migraine disease, as well as preparation method and application thereof
The invention belongs to the field of researches and development of traditional Chinese medicines, and particularly relates to a traditional Chinese medicine composition for preventing and treating a migraine disease. The composition is prepared by screening a formula of ligusticum wallichii and radix paeoniae alba based on traditional Chinese medical clinical experience, wherein the formula is obtained by means of solvent extraction and macroporous resin purification and enrichment, and mainly comprises ingredients of amino acids, phenolic acids, phthalides, terpenes and alkaloids. Pharmacodynamic experiments prove that the medicine composition can be used for reducing the content of 5-HT in plasma of a rat model, reducing the content of related peptide of calcitonin genes and reducing the content of endothelin, so that the migraine can be prevented and treated.
Owner:石任兵
Methods and compositions using substance p to promote wound healing
ActiveUS20100092561A1High activityEasy to explainOrganic active ingredientsBiocideSubstance KDense Core Vesicles
Owner:REID TED +1
Materials and Methods for Treatment of Inflammation
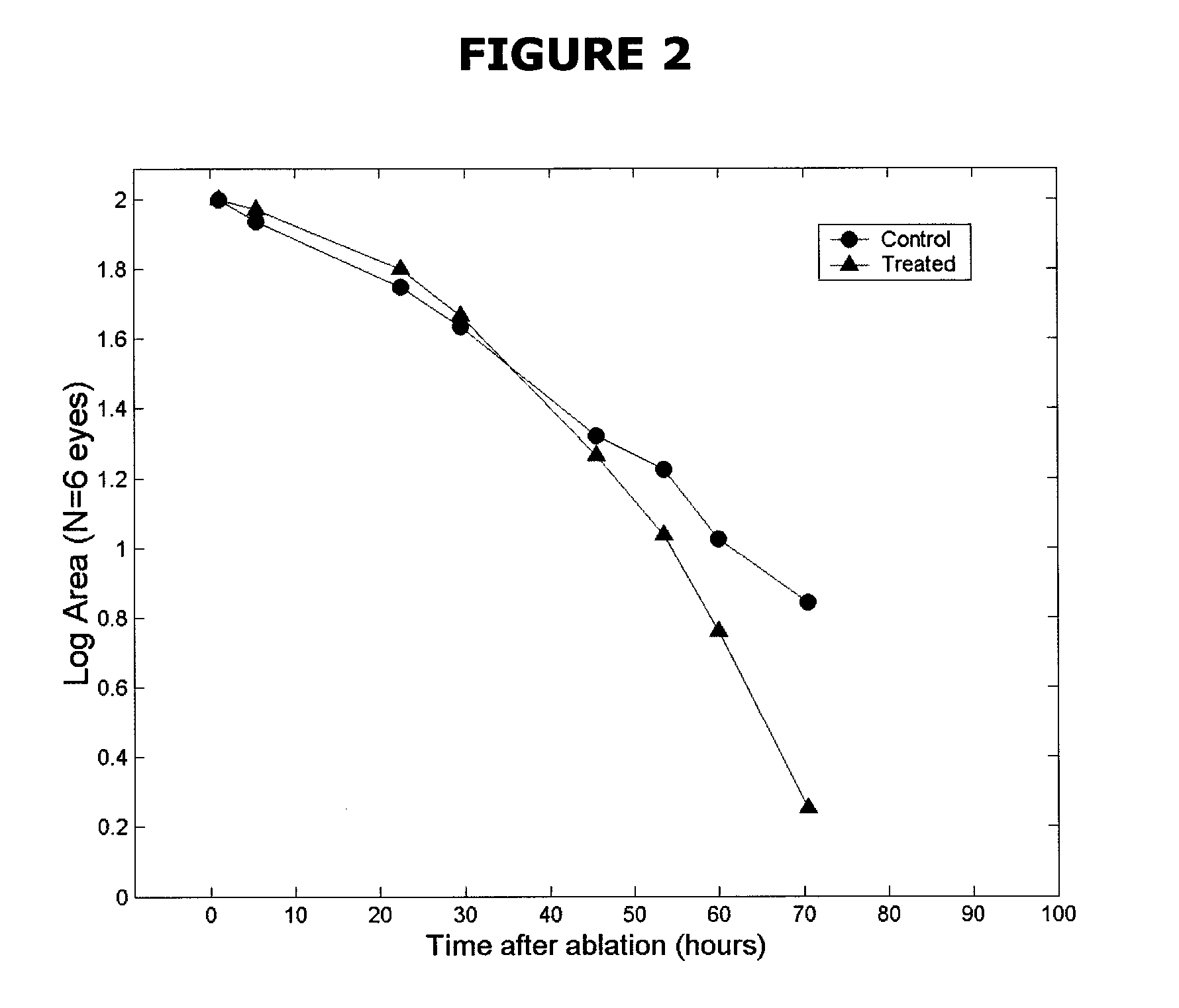
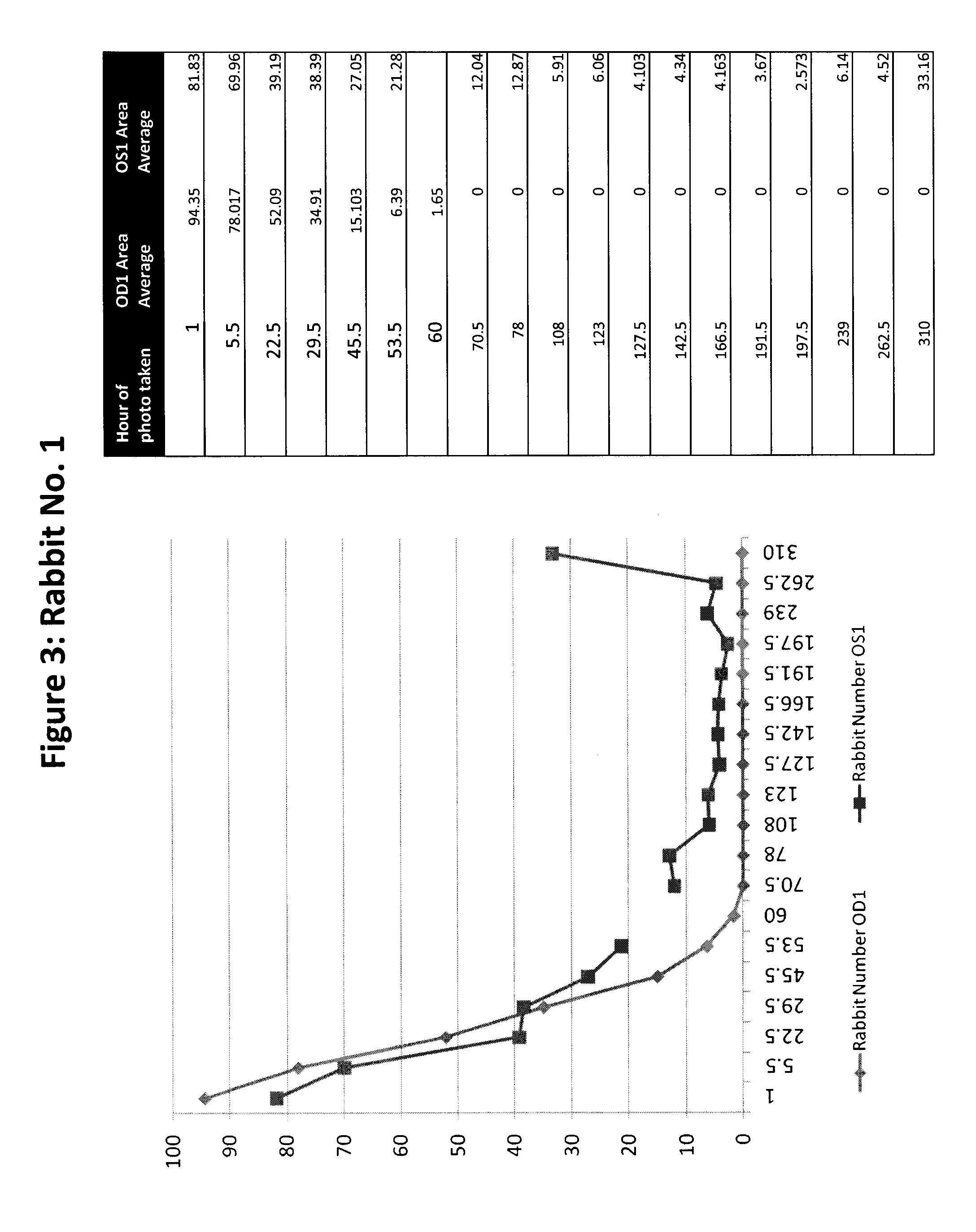
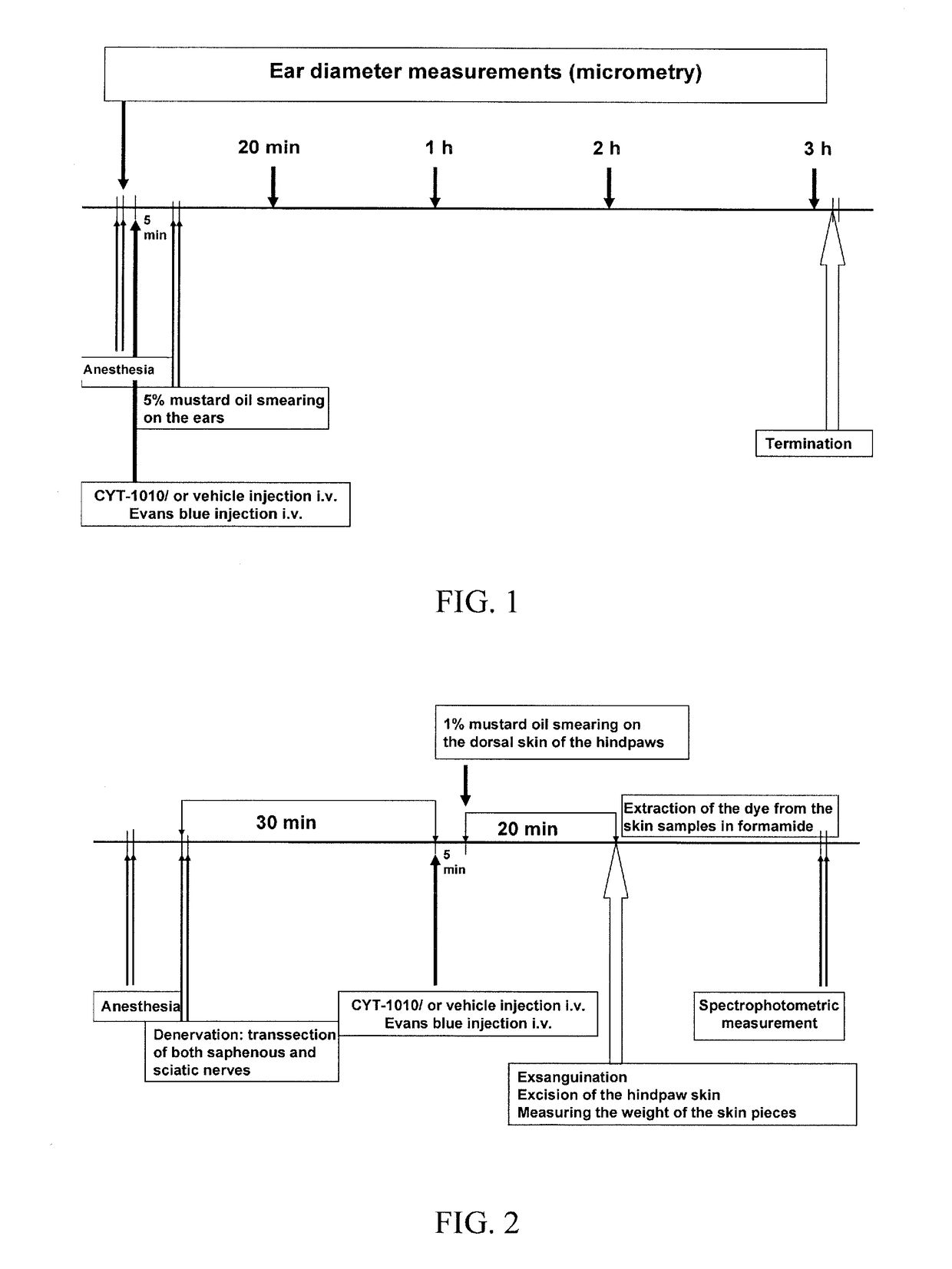
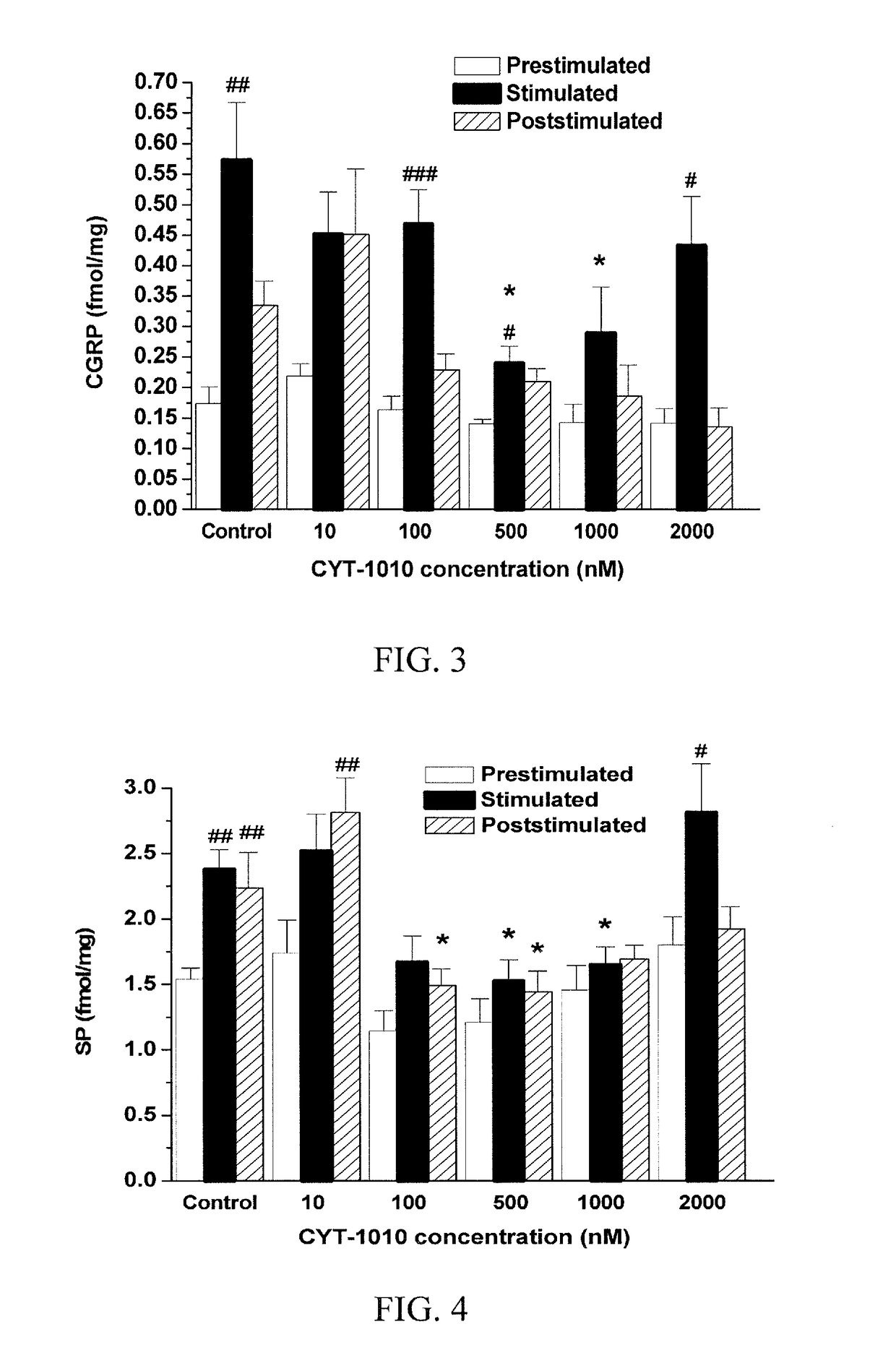
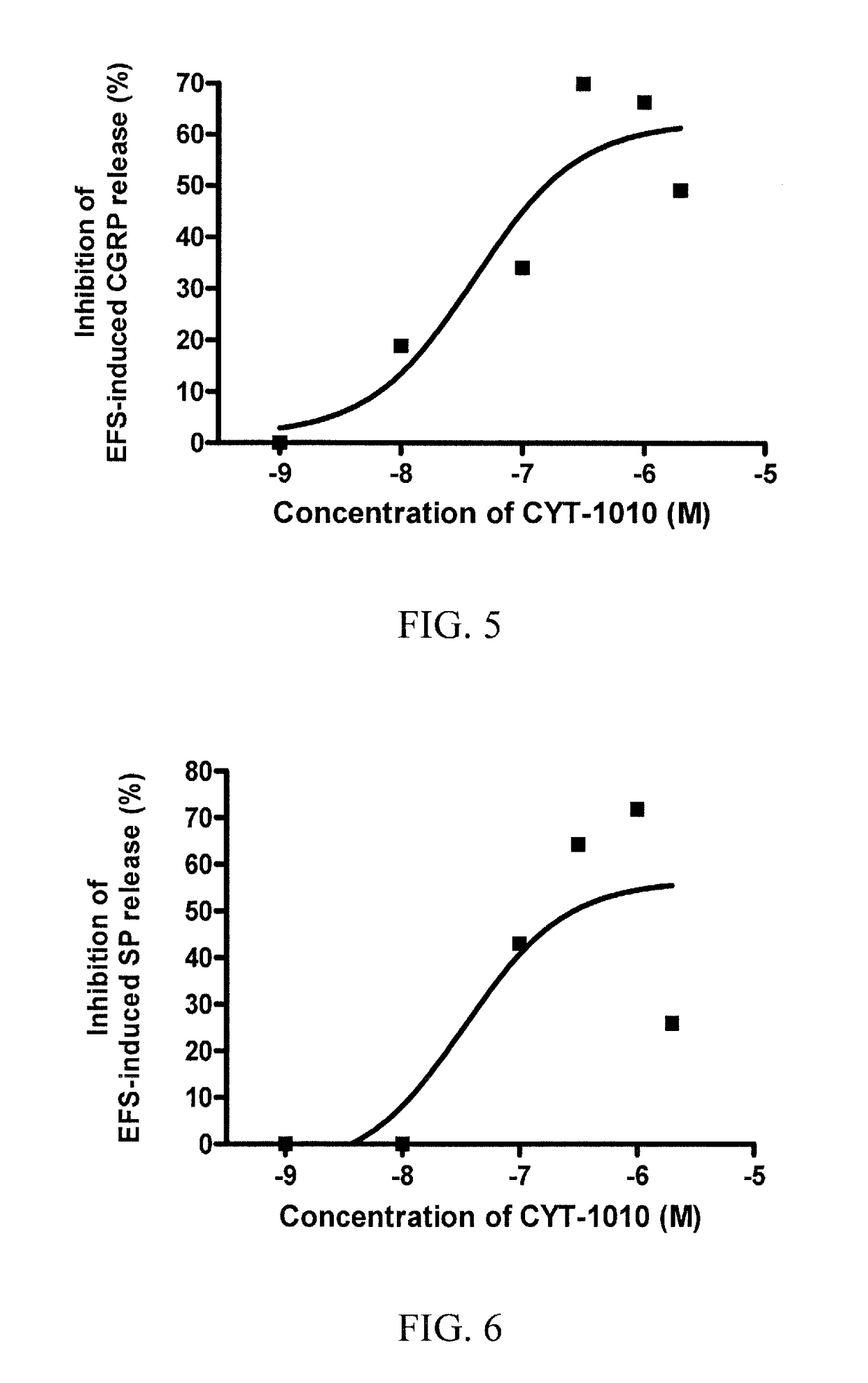
The subject invention pertains to peptides and salts thereof that are useful as anti-inflammatory agents and to compositions containing such peptides and salts as active ingredients. Specifically exemplified herein are endomorphin-1 peptide (EM-1), analogs and salts thereof, and uses for modulation of calcitonin gene-related peptide (CGRP) production and / or substance P (SP) and for treatment of inflammation, particularly neurogenic inflammation.
Owner:CYTOGEL PHARMA
Selecting headache patients responsive to antibodies directed against calcitonin gene related peptide
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com