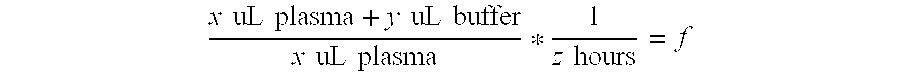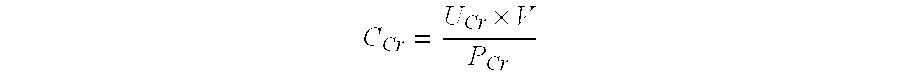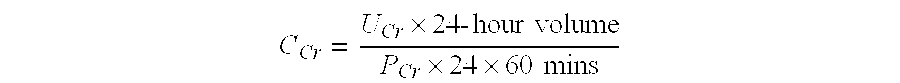Patents
Literature
66 results about "Angiotensinogenase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
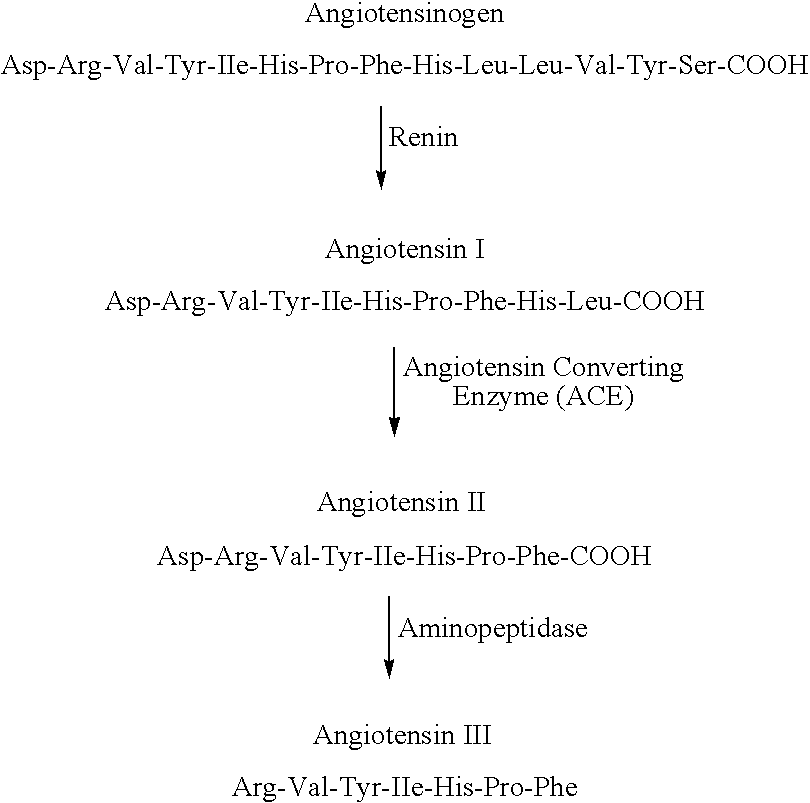
Renin (etymology and pronunciation), also known as an angiotensinogenase, is an aspartic protease protein and enzyme secreted by the kidneys that participates in the body's renin–angiotensin–aldosterone system (RAAS)—also known as the renin–angiotensin–aldosterone axis—that mediates the volume of extracellular fluid (blood plasma, lymph and interstitial fluid) and arterial vasoconstriction. Thus, it regulates the body's mean arterial blood pressure.
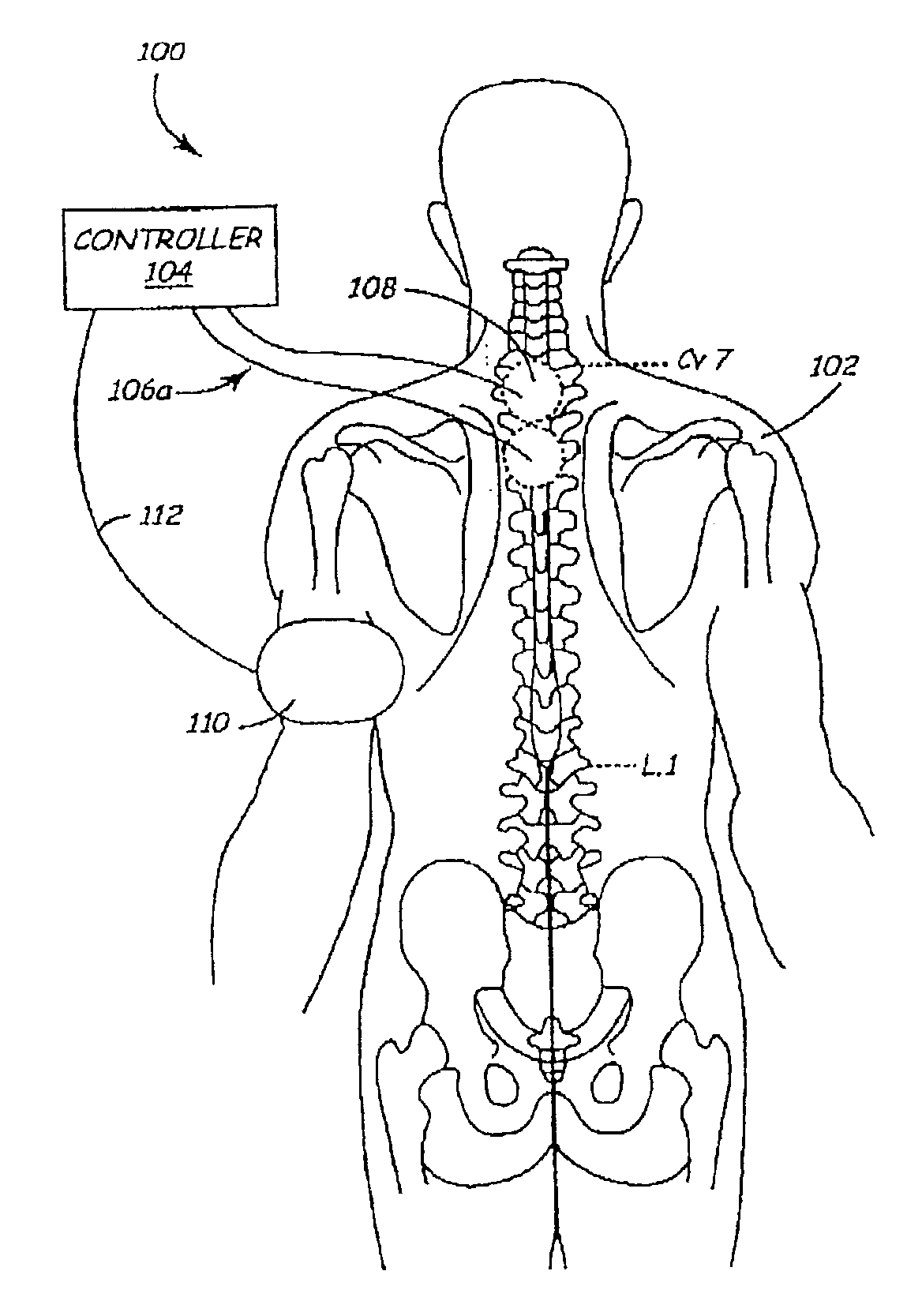
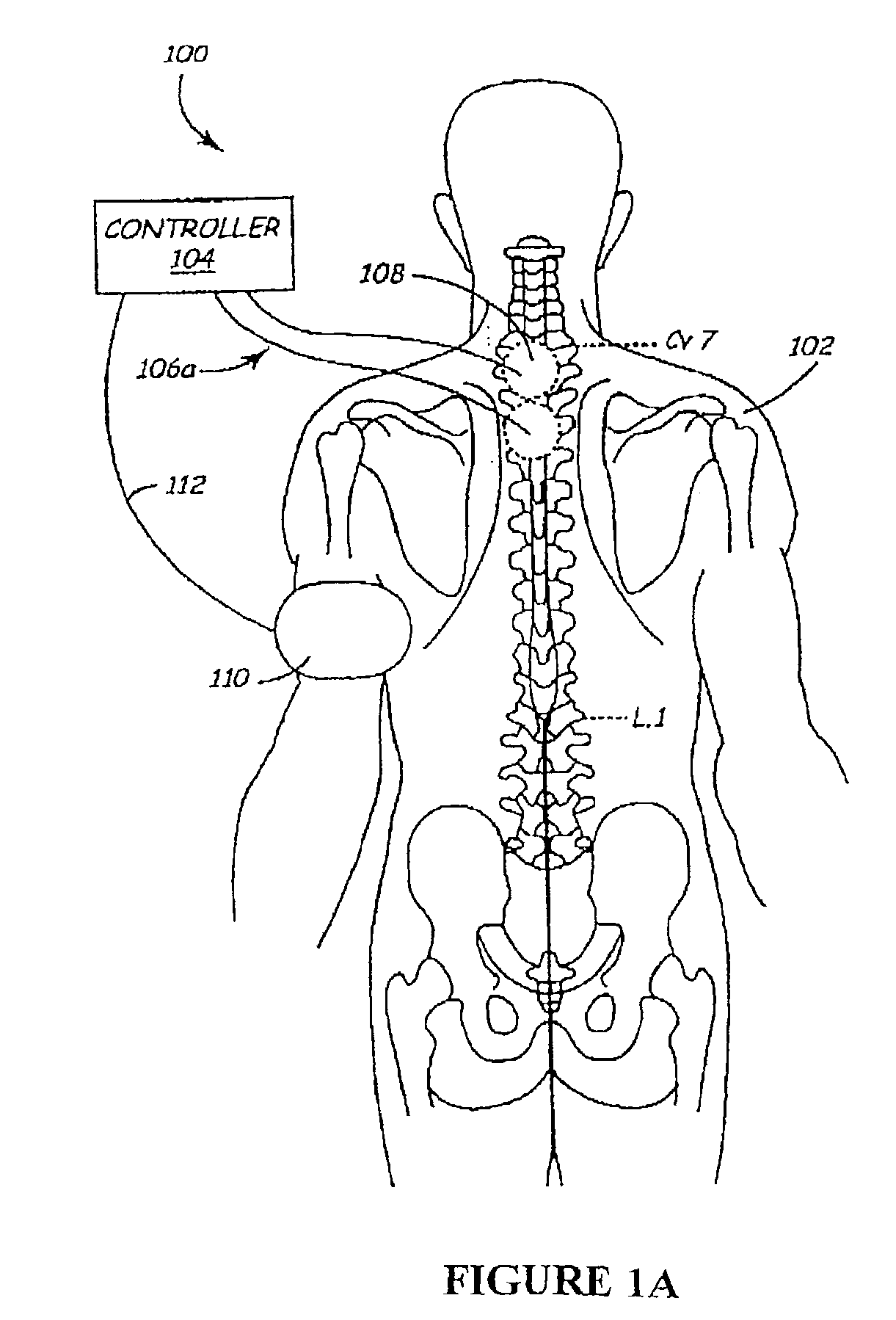
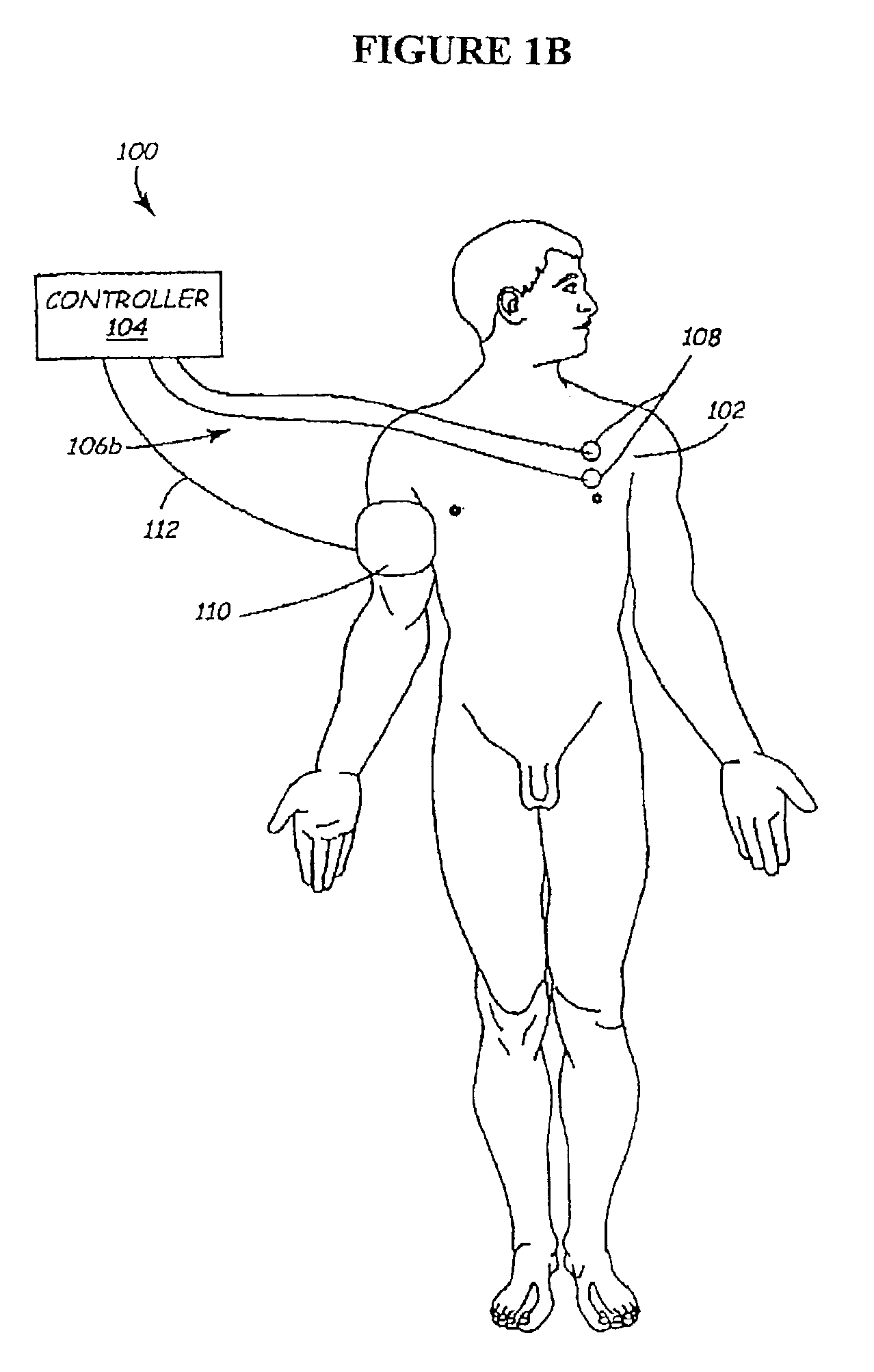
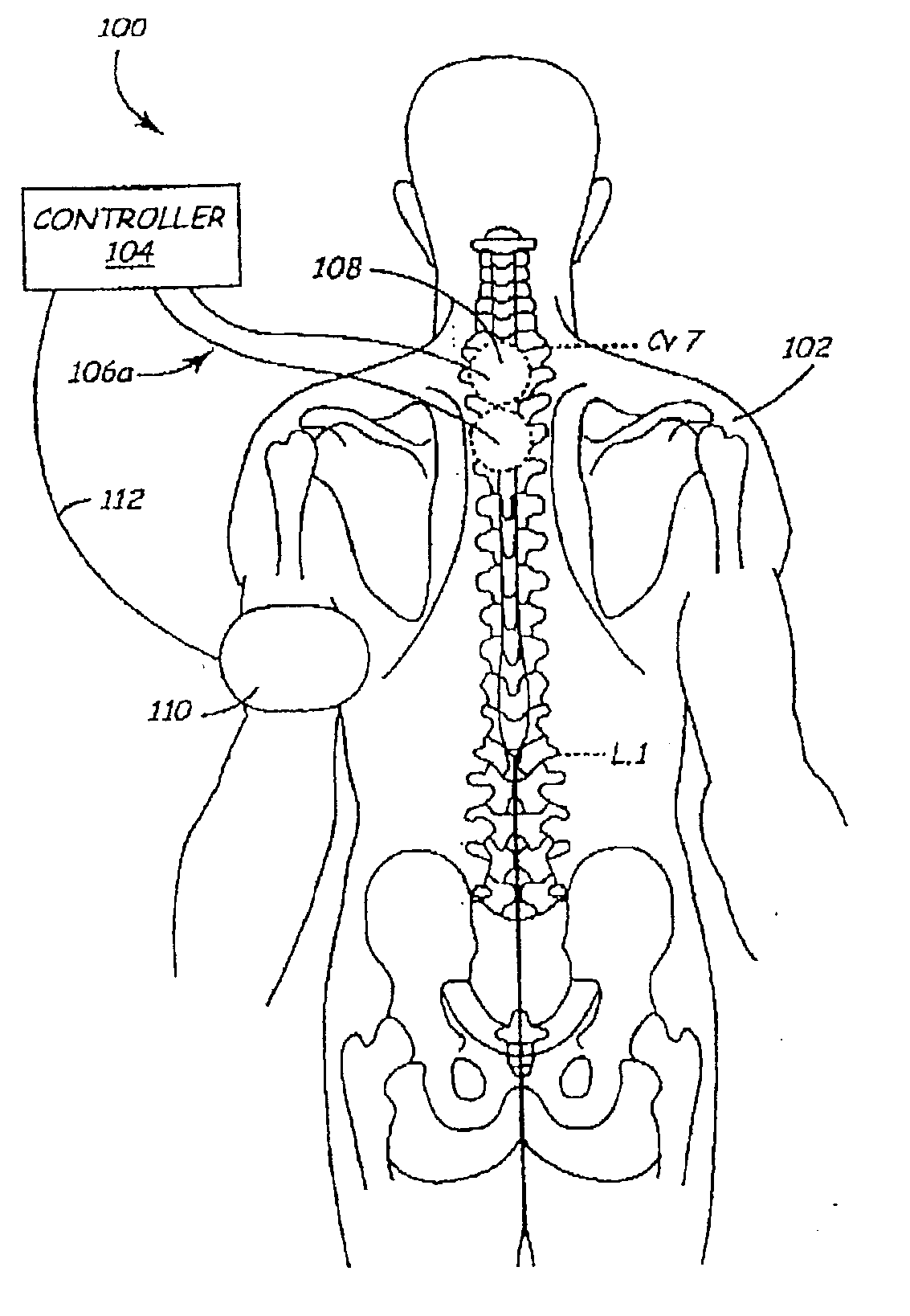
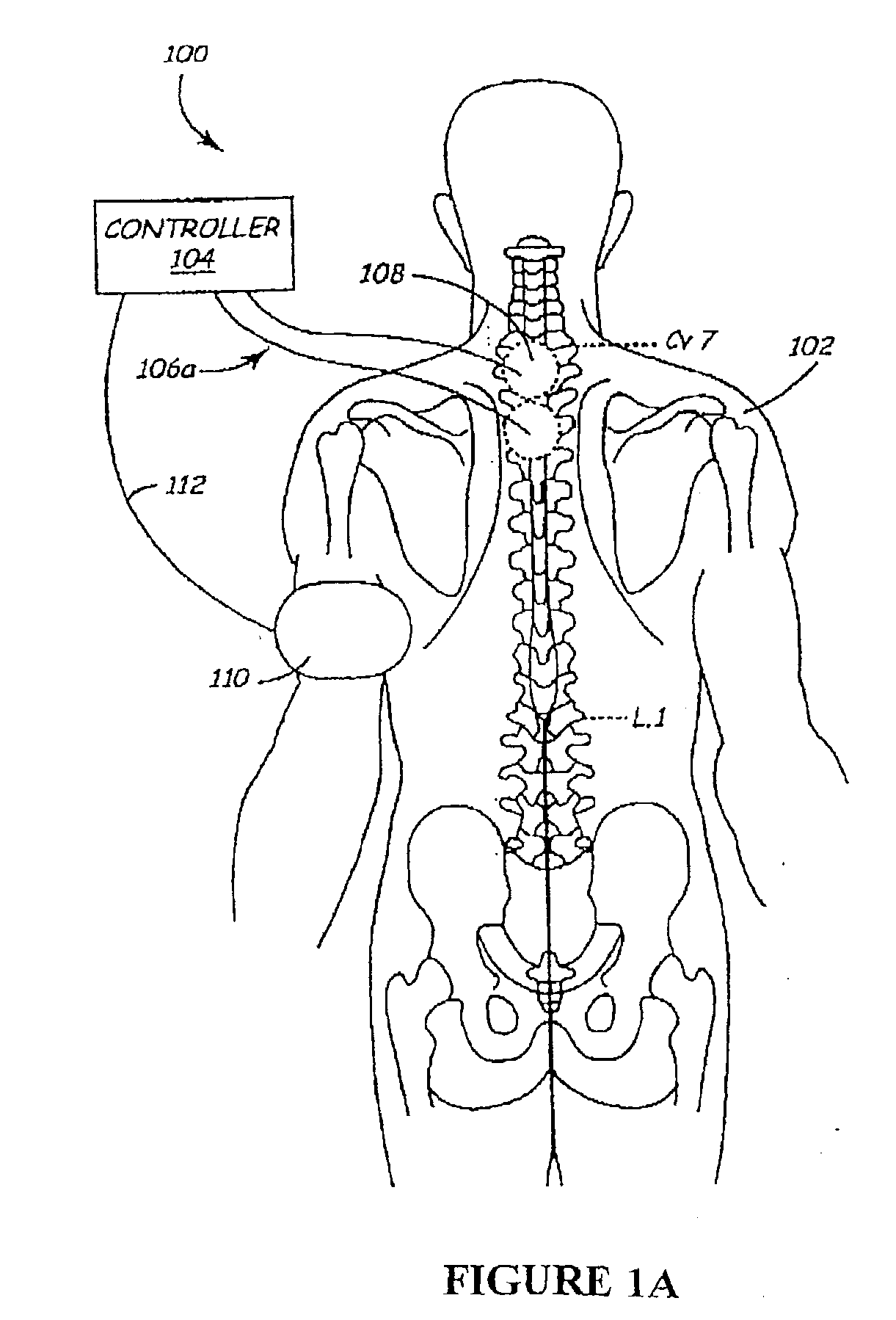
Methods and apparatus for the regulation of hormone release
ActiveUS7221979B2Modulate hormone levelPrevent hormone imbalancesInternal electrodesExternal electrodesCatecholamineElectrical stimulations
A method and apparatus for delivering corrective therapy through hormone regulation is provided. Inhibition of sympathetic fibers by spinal cord stimulation is used to regulate the levels of hormones such as catecholamines, renin, and calcitonin gene-related peptide. The invention utilizes a closed or open loop feedback system in which physiological parameters such as the concentrations of hormones and sympathetic indicators such as heart rate and urine production are monitored and used to determine the appropriate level of neurostimulation. The site of electrical stimulation includes, but is not limited to, the spinal cord at levels T7–L2 and the associated neural fibers within a region of the T7–L2 dermatomes
Owner:MEDTRONIC INC
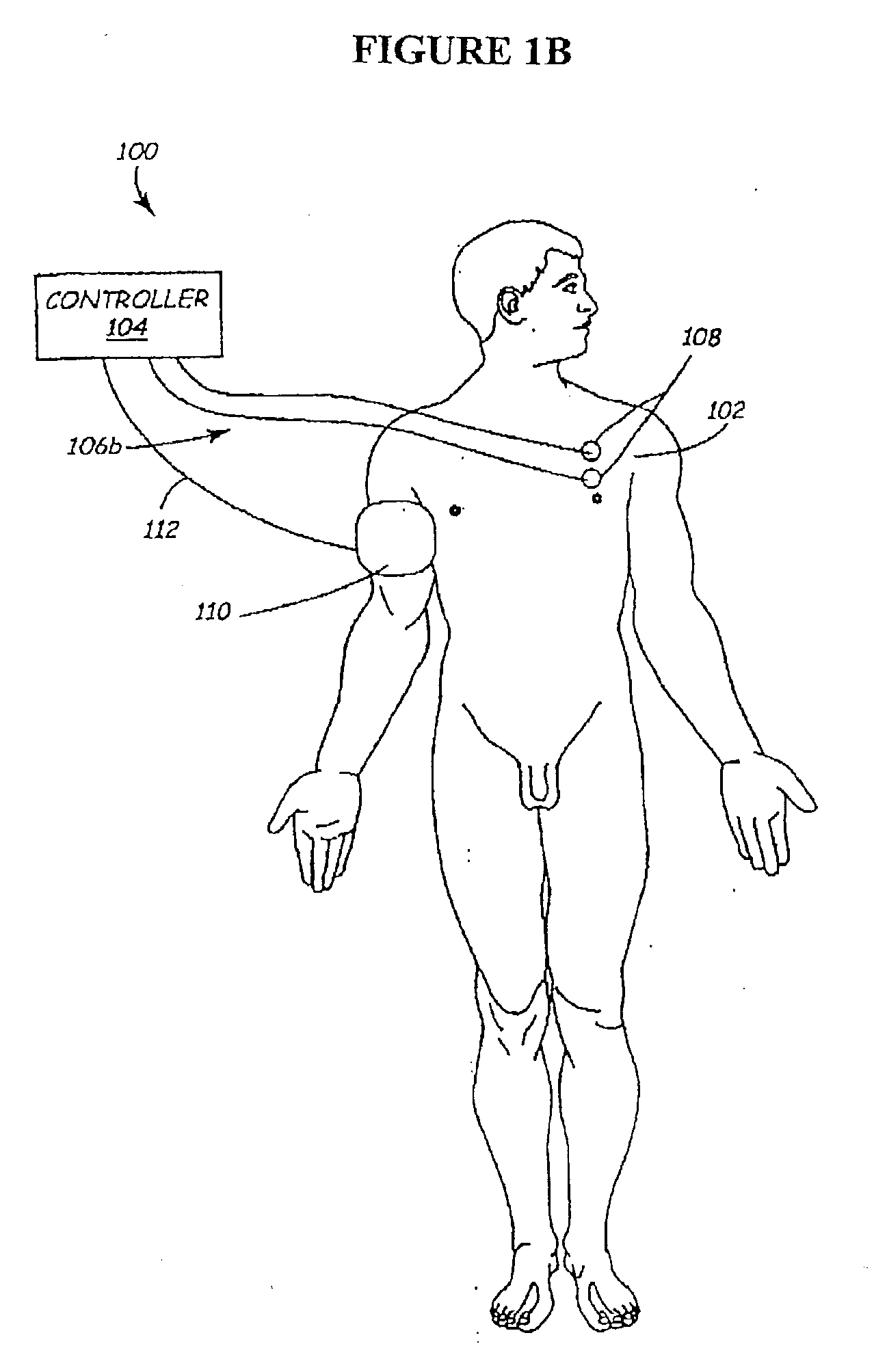
Methods and apparatus for the regulation of hormone release
A method and apparatus for delivering corrective therapy through hormone regulation is provided. Inhibition of sympathetic fibers by spinal cord stimulation is used to regulate the levels of hormones such as catecholamines, renin, and calcitonin gene-related peptide. The invention utilizes a closed or open loop feedback system in which physiological parameters such as the concentrations of hormones and sympathetic indicators such as heart rate and urine production are monitored and used to determine the appropriate level of neurostimulation. The site of electrical stimulation includes, but is not limited to, the spinal cord at levels T7-L2 and the associated neural fibers within a region of the T7-L2 dermatomes
Owner:MEDTRONIC INC
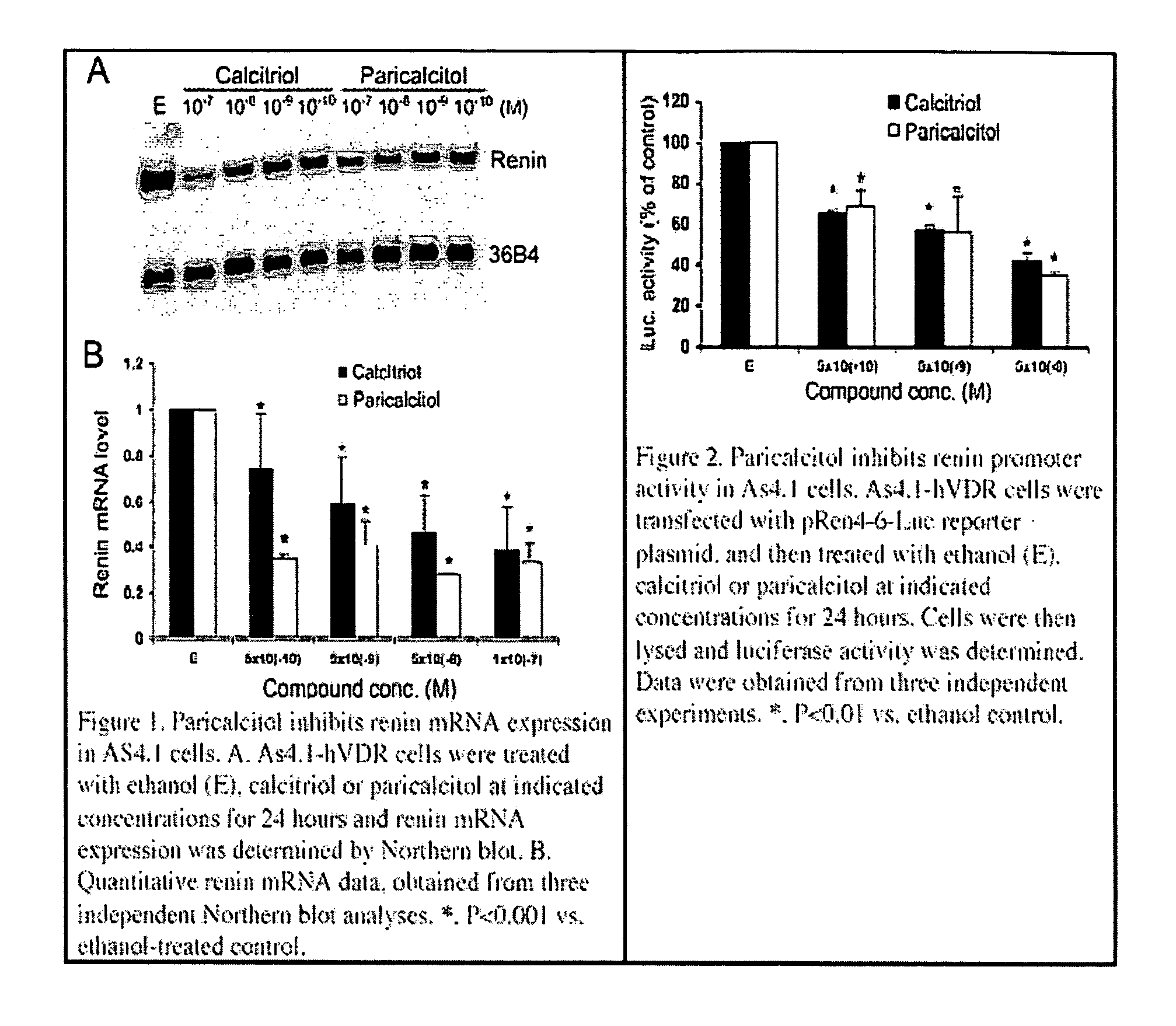
Use of vitamin Ds to treat kidney disease
InactiveUS20050124591A1Reduce inflammationSuppression problemBiocideUrinary disorderDiseaseAngiotensin Receptor Blockers
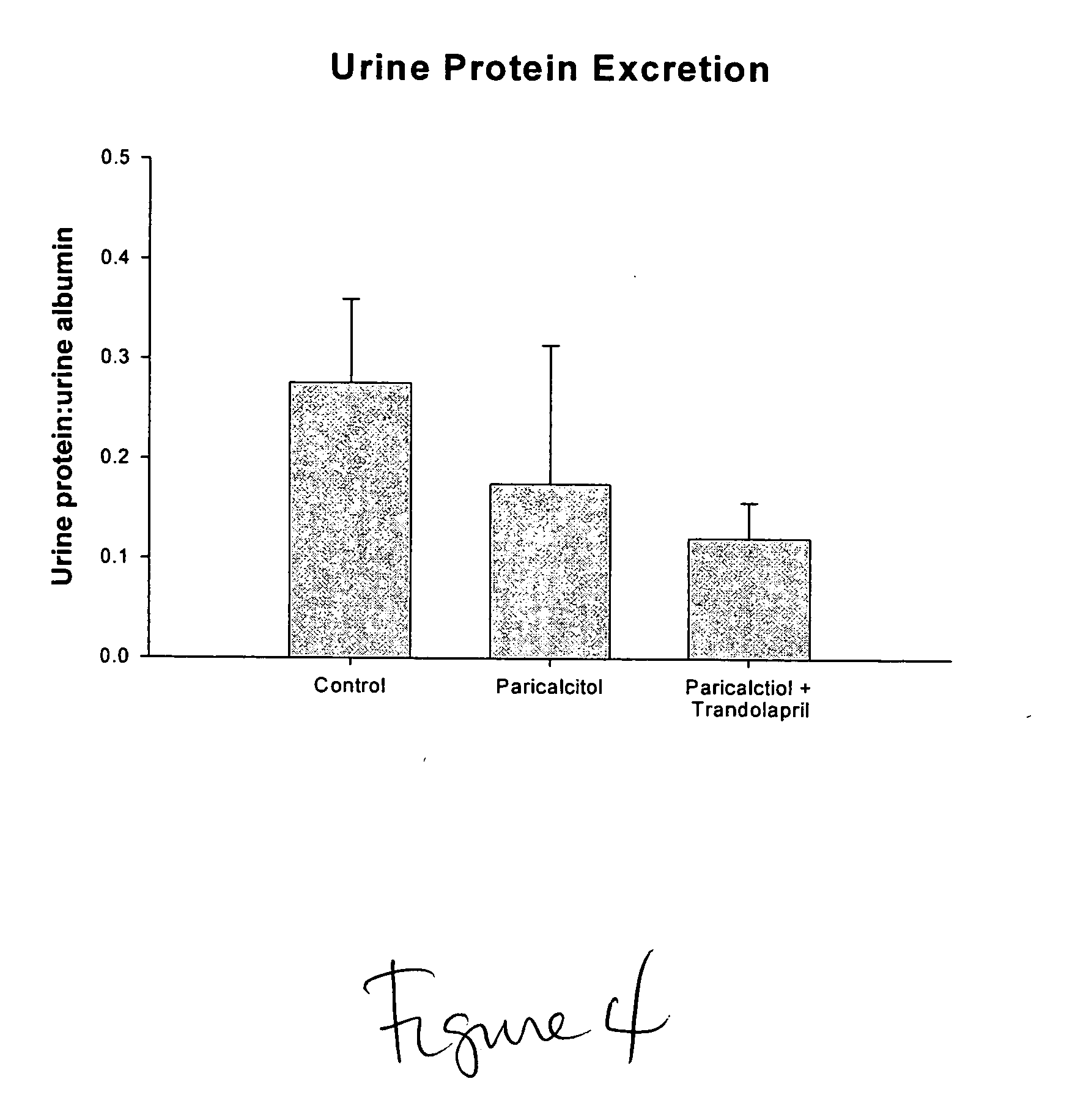
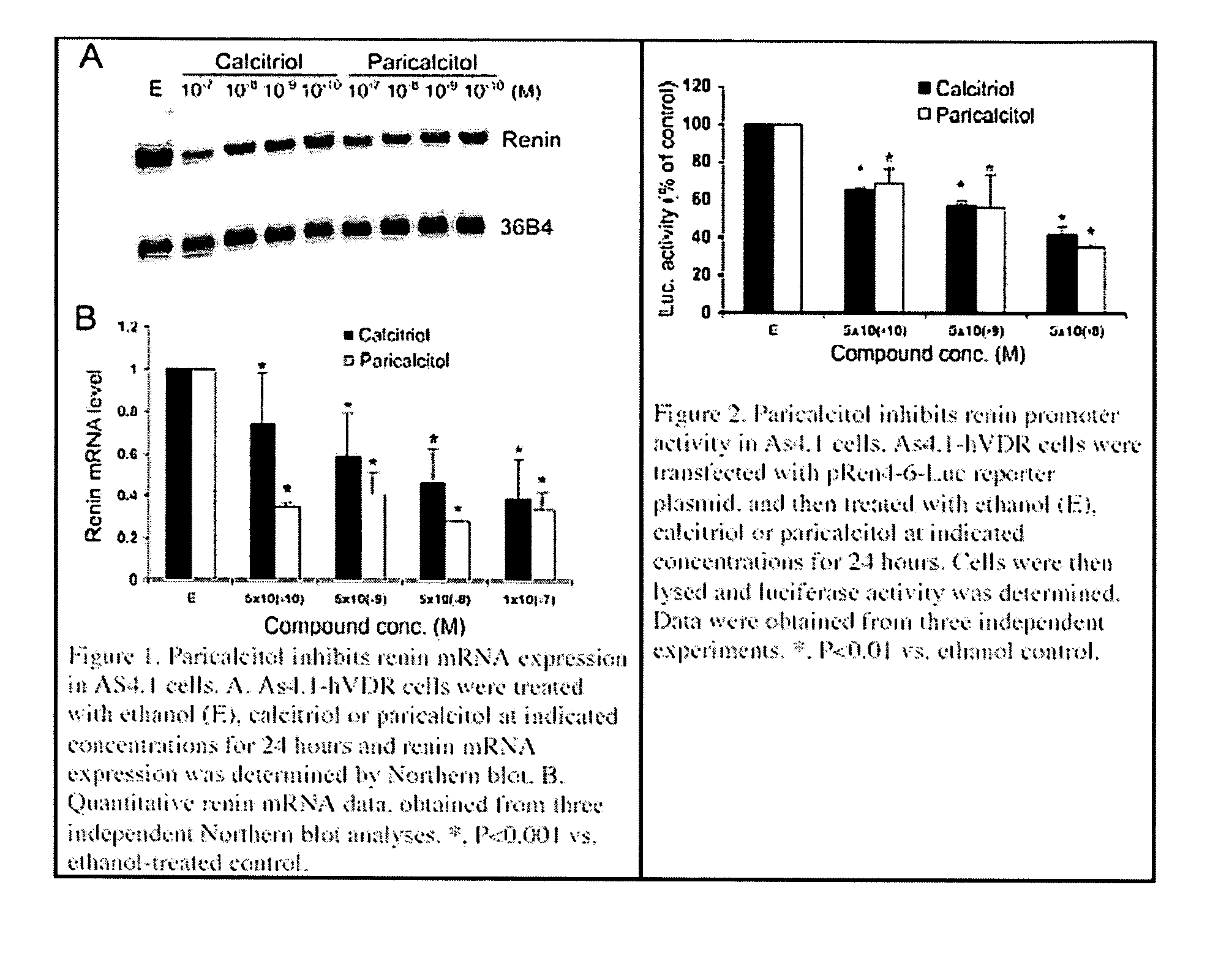
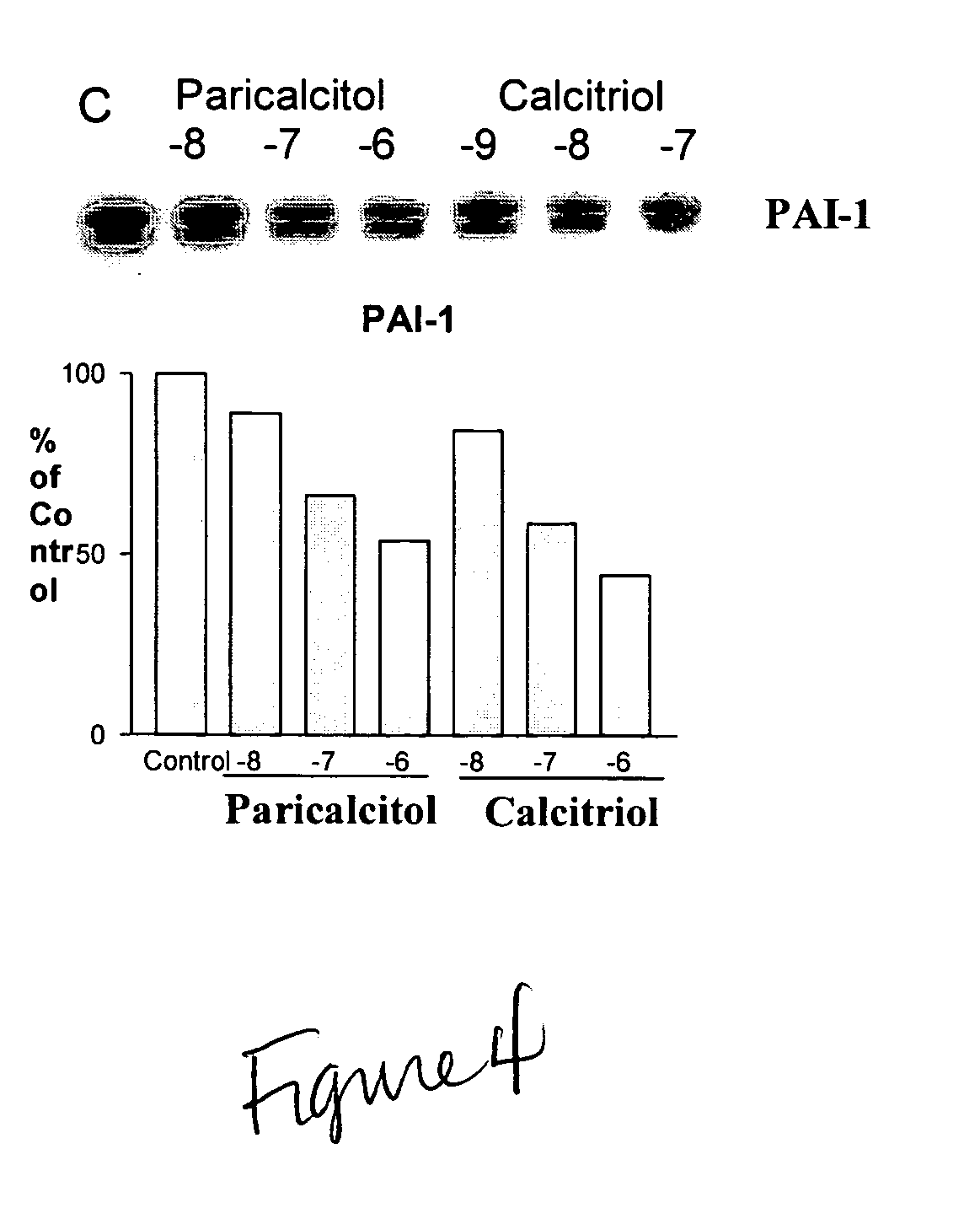
Disclosed are compositions containing a VDRA / Vitamin D analog to treat or prevent kidney disease, including chronic kidney disease. The present invention also relates to methods of treating kidney disease by administering to a patient a pharmaceutical composition containing a therapeutically effective amount of a VDRA / Vitamin D analog. Compositions according to the invention include a VDRA / Vitamin D analog and at least one of the following agents: an ACE inhibitor, an angiotensin (II) receptor blocker (ARB) and aldosterone blocker in therapeutically effective amounts to inhibit renin production or inhibit activation of the renin-angiotensin-aldosterone system. Preferred compositions contain paricalcitol with at least one of these other agents. Such combinations can avoid ACE inhibition escape and aldosterone escape with subsequent increase in angiotensin (II) and aldosterone generation.
Owner:ABBOTT LAB INC
Treatment of Prevention of Unscheduled Bleeding in Women on Progestogen Containing Medication
InactiveUS20090197843A1Increased chronotropismIncreased inotropismBiocidePeptide/protein ingredientsPhysiologyProgestogen
The present invention relates to a method of treating or preventing unscheduled bleeding in women, the unscheduled bleeding being the result of repeated administration of a hormonal composition that contains a progestogen, wherein the method includes the administration of an effective amount of Renin Angiotensin System (RAS) suppressor selected from angiotensin converting enzyme inhibitors; angiotensin II receptor antagonists; renin inhibitors and combinations thereof. Other aspects of the invention relate to a pharmaceutical composition containing a RAS suppressor and a progestogen and to a pharmaceutical kit having a plurality of dosage units, wherein at least one dosage unit contains a progestogen; at least one dosage unit contains an estrogen; and at least one dosage unit contains a RAS suppressor.
Owner:PANTARHEI BIOSCI
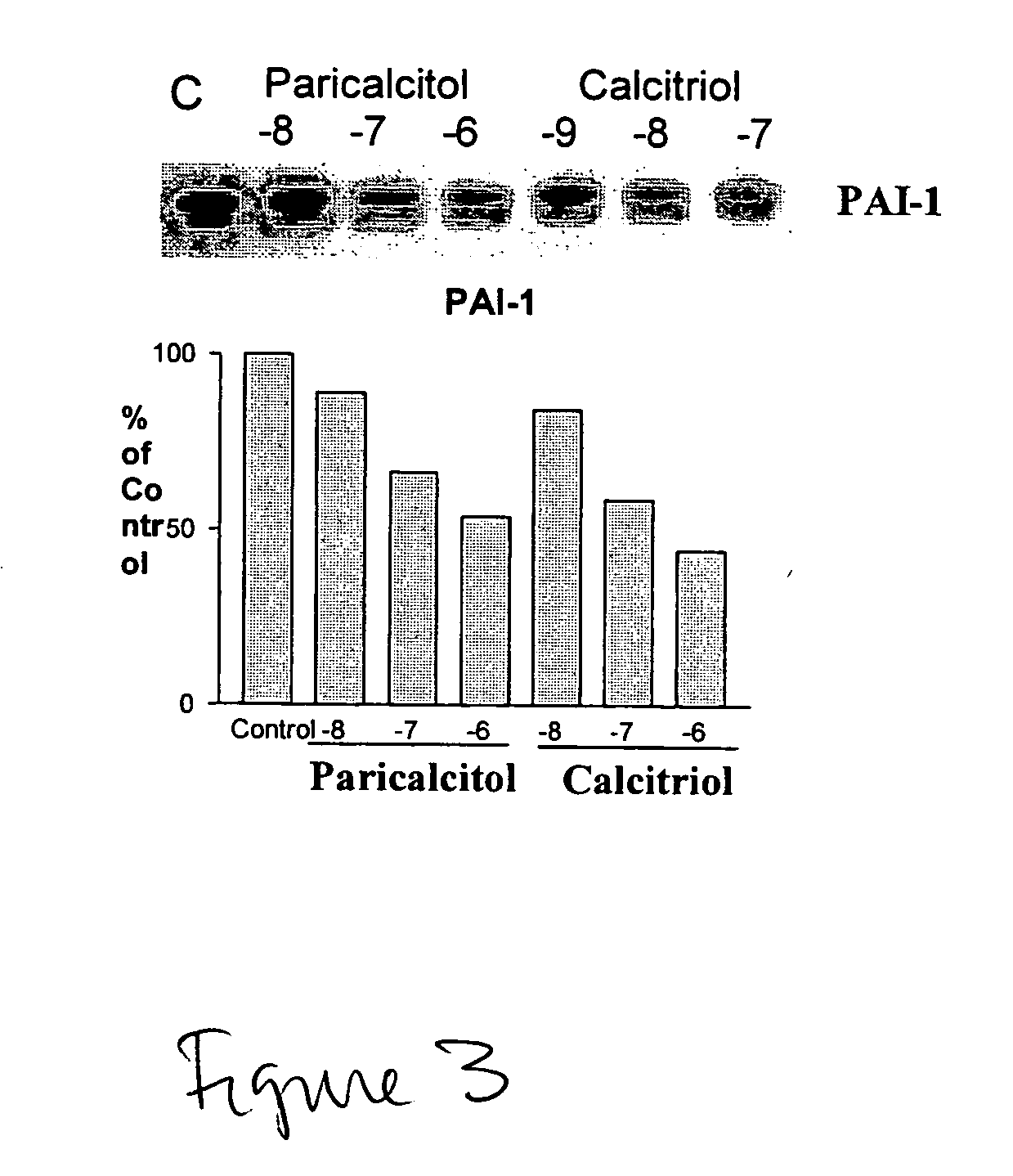
Use of Vitamin Ds to treat kidney disease
InactiveUS20050148557A1Reduce inflammationSuppression problemBiocideUrinary disorderDiseaseAldosterone escape
Disclosed are compositions containing a VDRA / Vitamin D analog to treat or prevent kidney disease, including chronic kidney disease. The present invention also relates to methods of treating kidney disease by administering to a patient a pharmaceutical composition containing a therapeutically effective amount of a VDRA / Vitamin D analog. Compositions according to the invention include a VDRA / Vitamin D analog and at least one of the following agents: an ACE inhibitor, an angiotensin (II) receptor blocker (ARB) and aldosterone blocker in therapeutically effective amounts to inhibit renin production or inhibit activation of the renin-angiotensin-aldosterone system. Preferred compositions contain paricalcitol with at least one of these other agents. Such combinations can avoid ACE inhibition escape and aldosterone escape with subsequent increase in angiotensin (II) and aldosterone generation.
Owner:UNIVERSITY OF CHICAGO
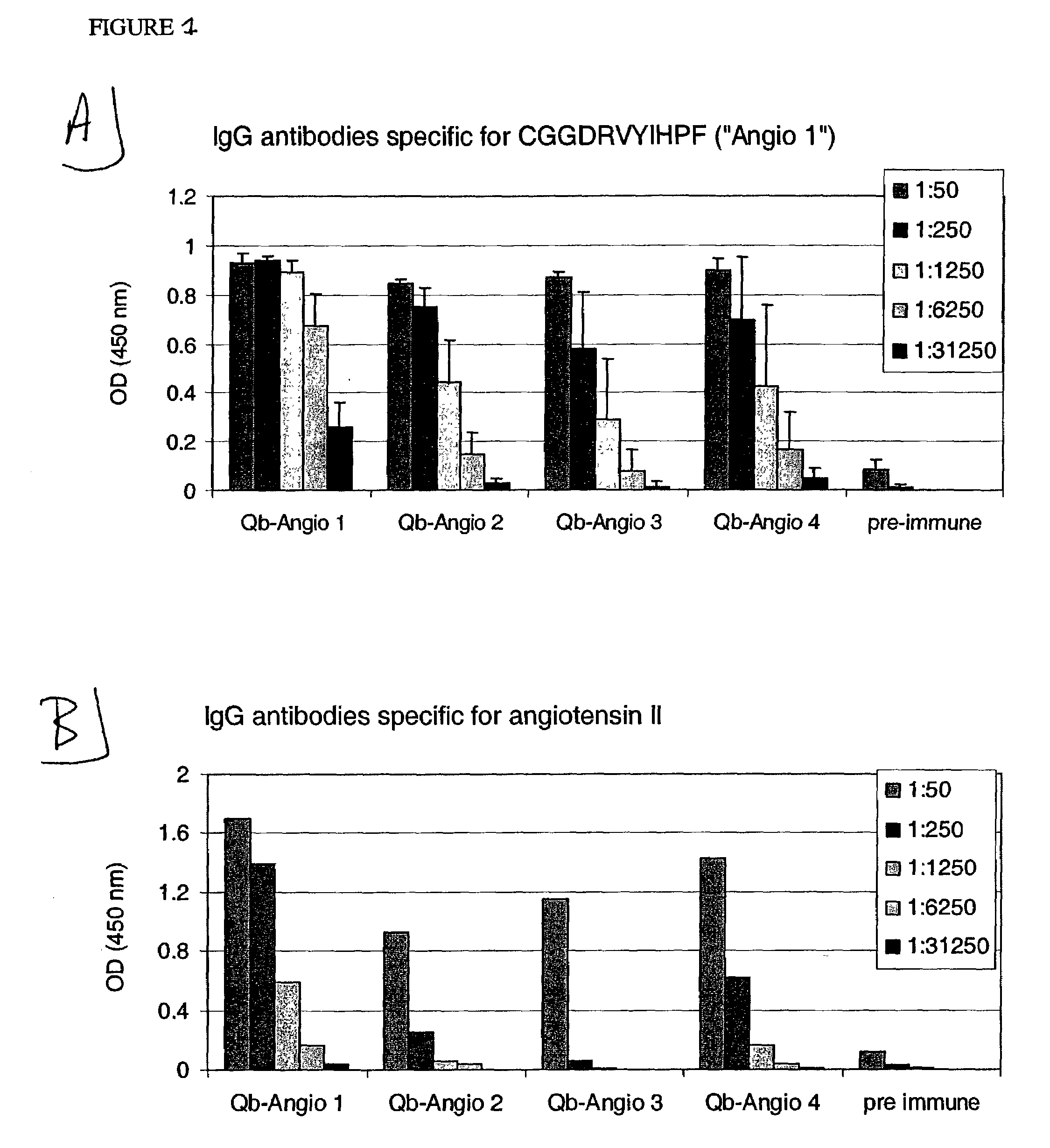
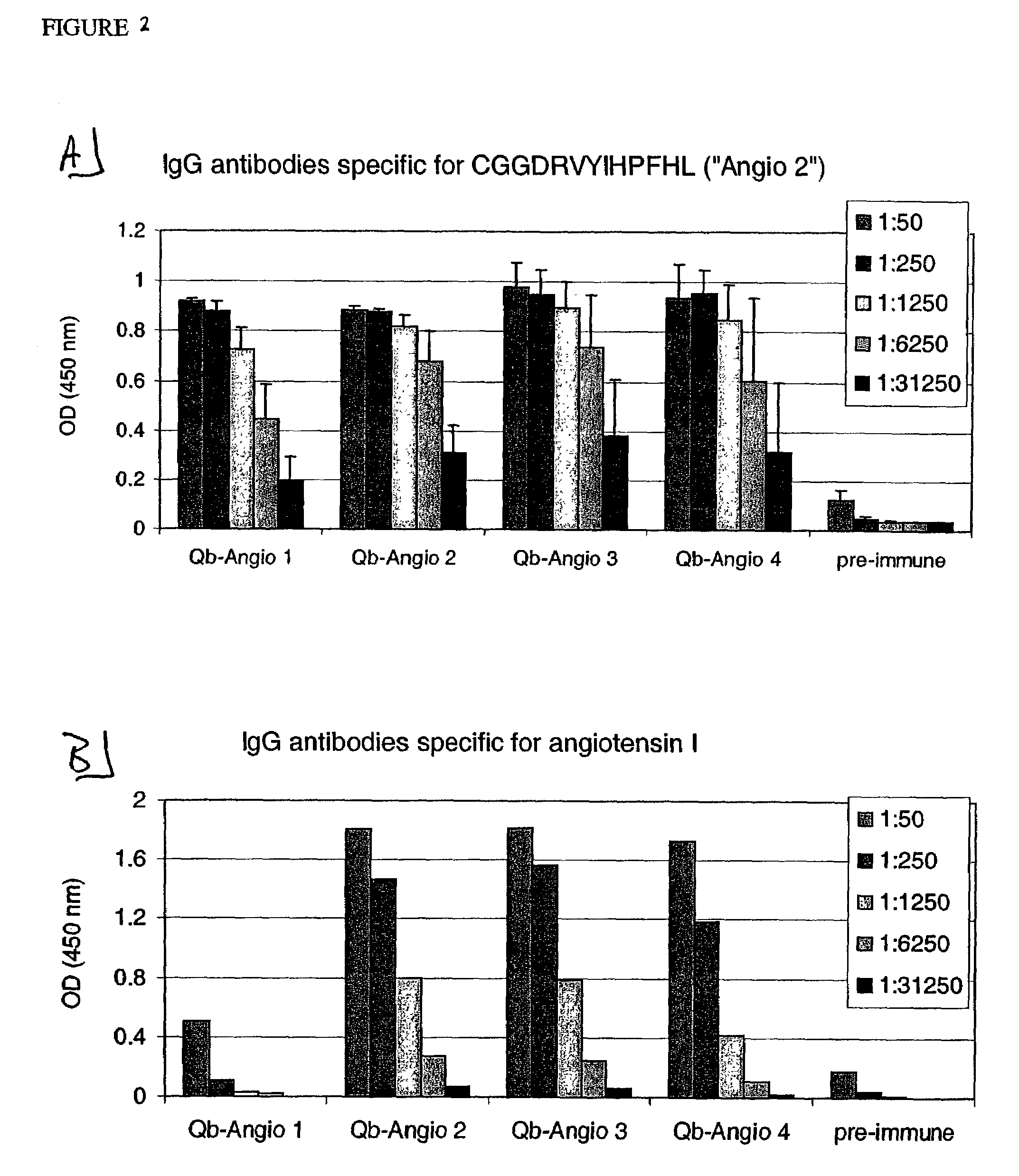
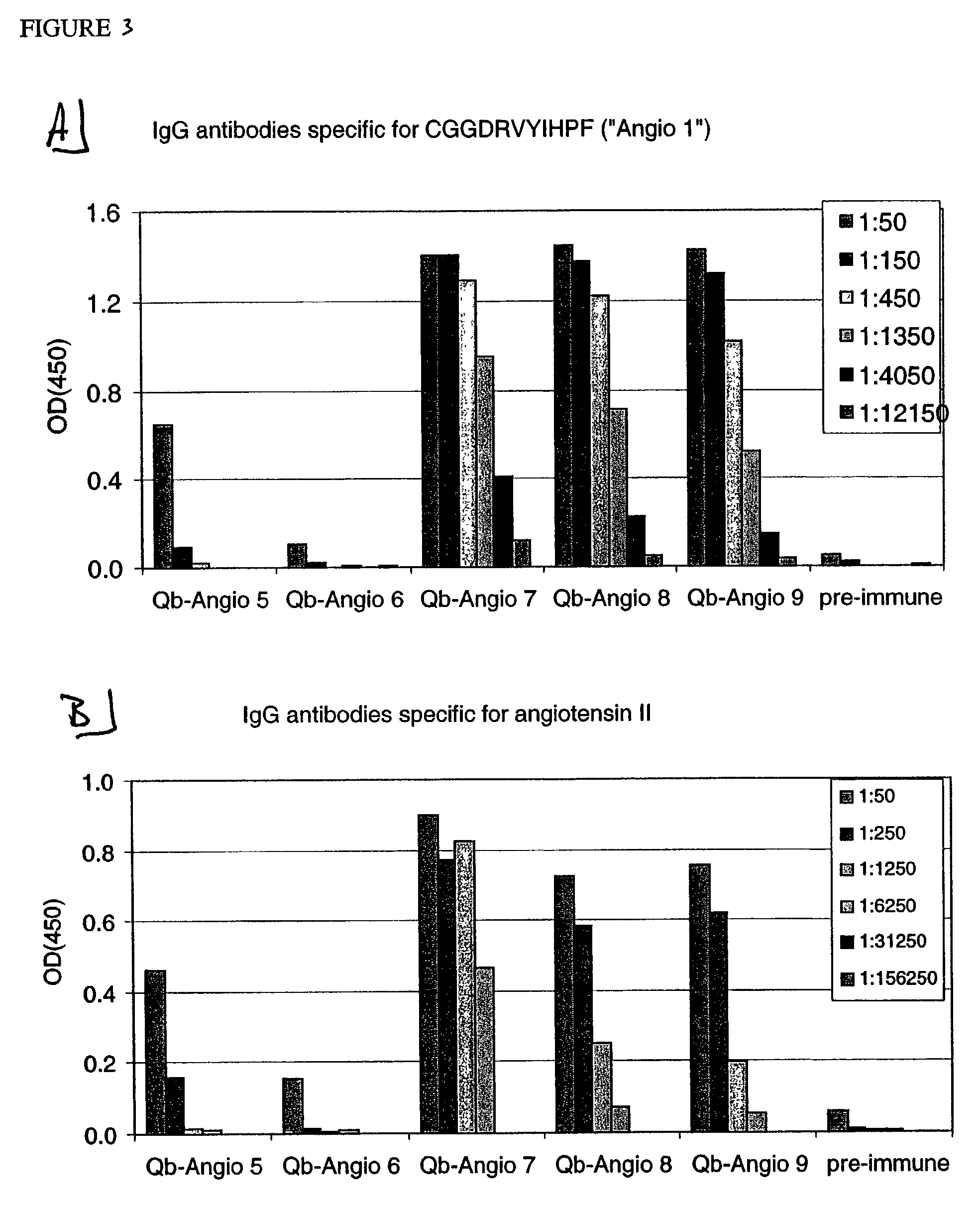
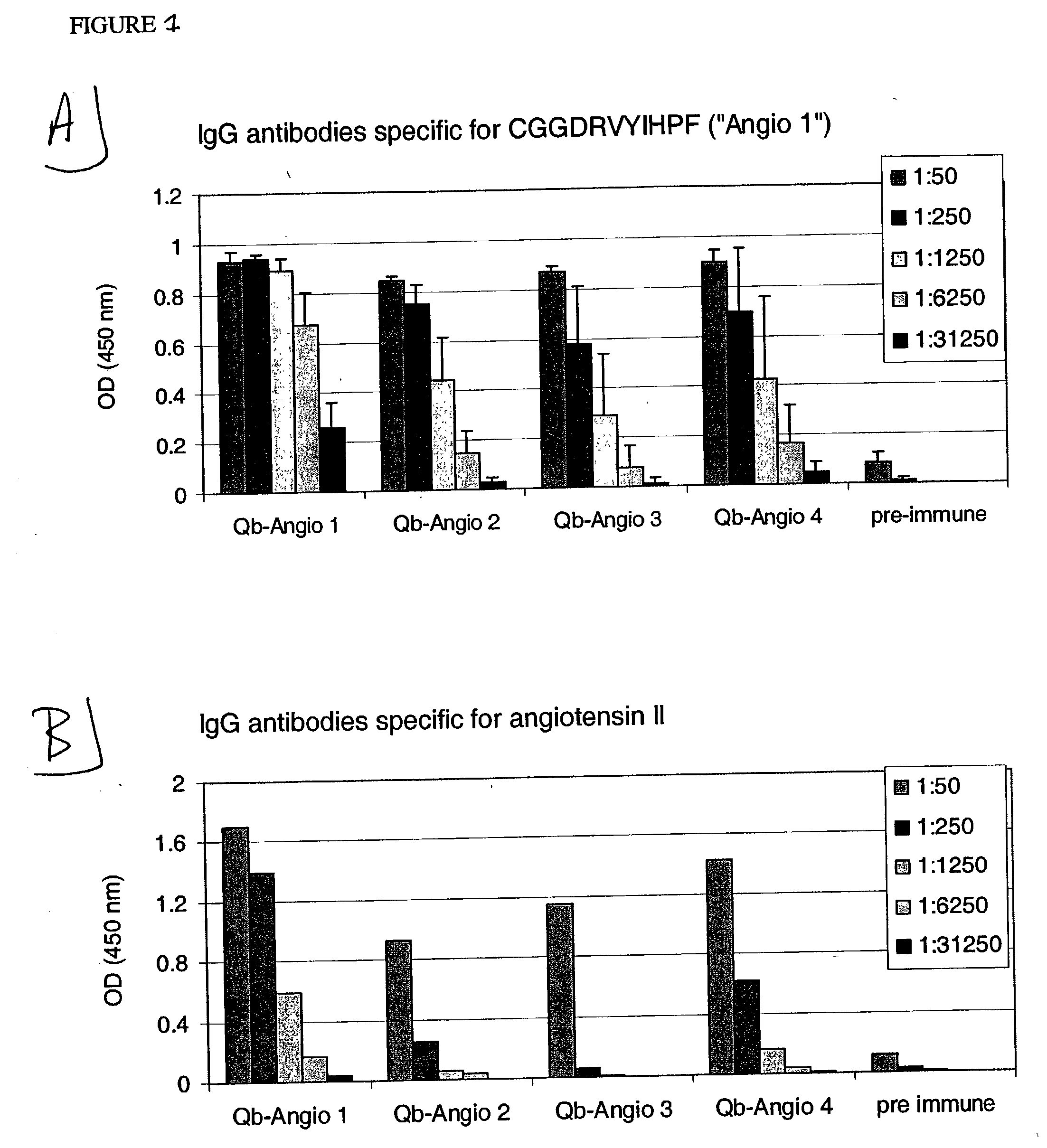
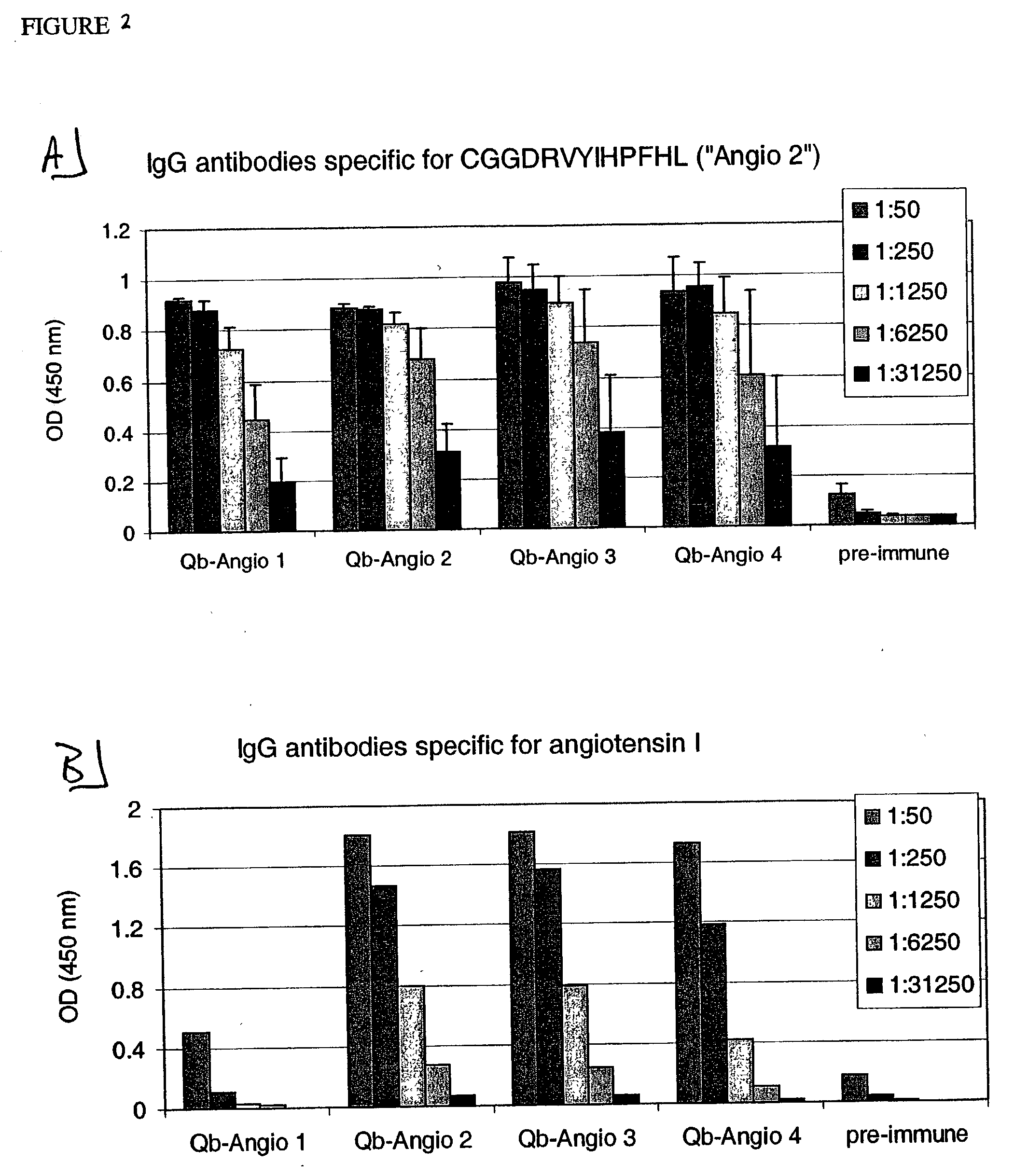
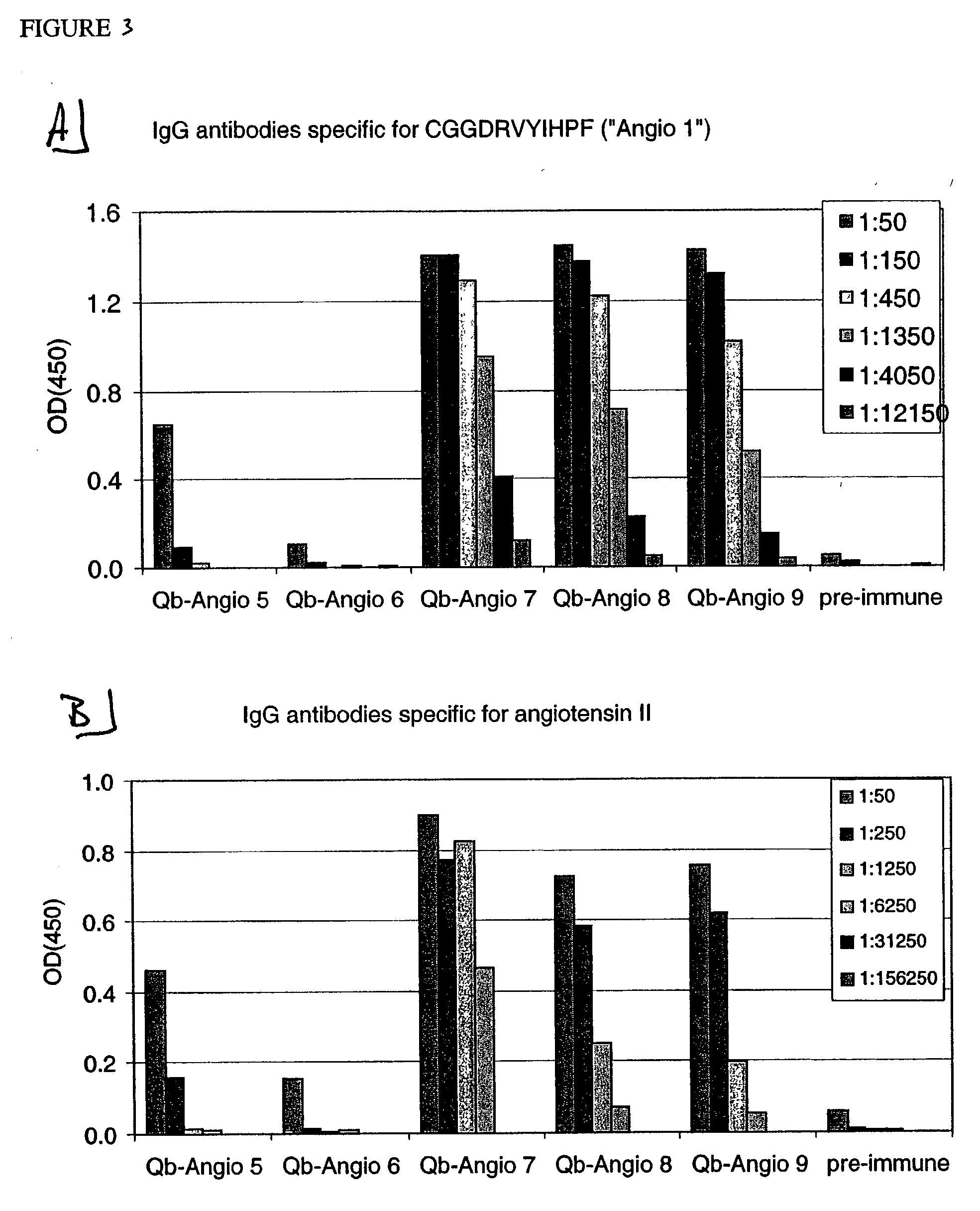
Angiotensin peptide-carrier conjugates and uses thereof
InactiveUS7115266B2Promote formationImprove complianceVirusesPeptide/protein ingredientsVirus-like particleAngiotensinogen mrna
The present invention provides conjugates of peptide derivatives of the mammalian peptide hormones angiotensinogen, angiotensin I and angiotensin II, presented in a repetitive scaffold by coupling the peptide derivatives to a carrier, particularly a virus-like particle (VLP). The invention also provides methods of producing such conjugates, and immunotherapeutic uses of the resulting immunogen conjugates for the therapy and prophylaxis of conditions associated with the renin-activated angiotensin system.
Owner:CYTOS BIOTECHNOLOGY AG
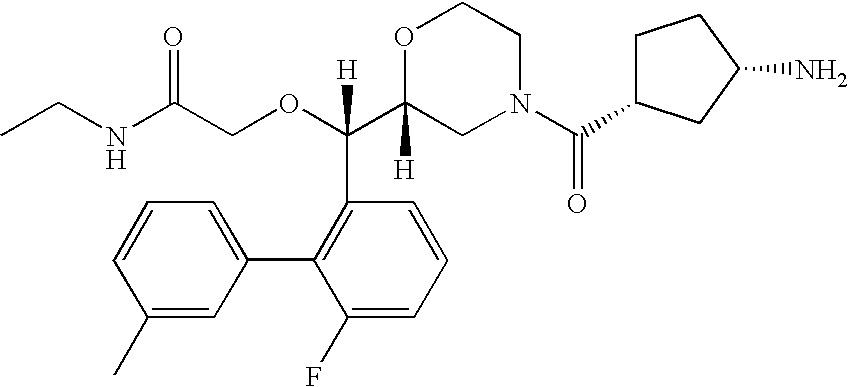
Renin inhibitors
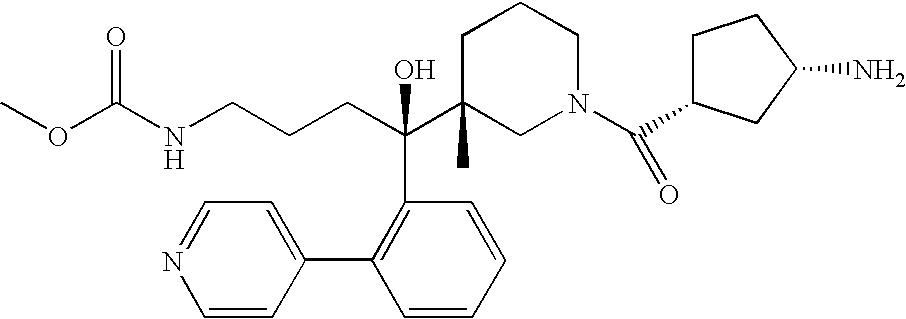
Disclosed are aspartic protease inhibitors represented by the following structural formula: and pharmaceutically acceptable salts thereof. These compounds are orally active and bind to aspartic proteases to inhibit their activity. They are useful in the treatment or amelioration of diseases associated with aspartic protease activity. The present invention is also directed to pharmaceutical compositions comprising a compound described herein or enantiomers, diastereomers, or salts thereof and a pharmaceutically acceptable carrier or excipient.
Owner:VITAE PHARMA INC
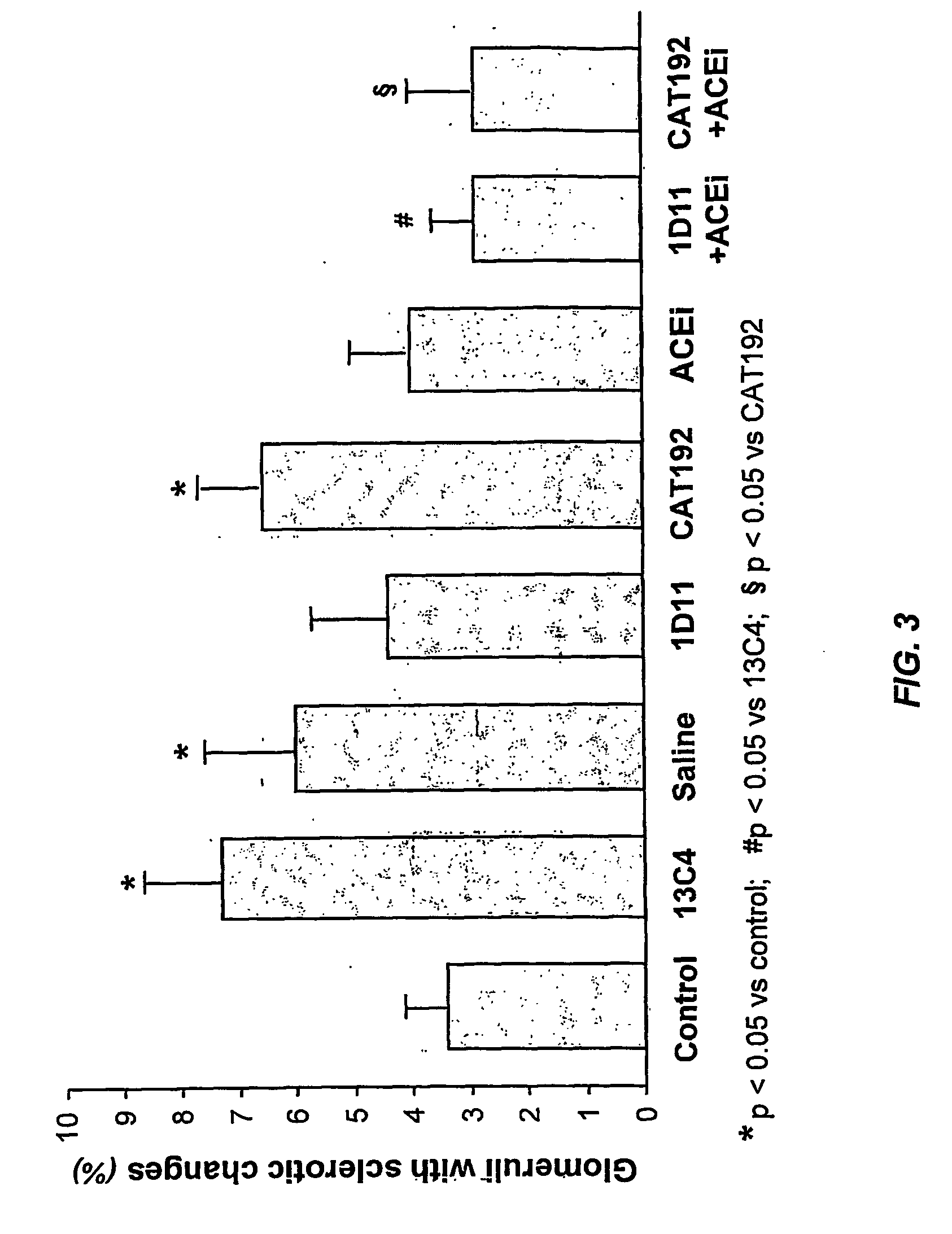
Tgf-beta antagonists combined with renin-angiotensin-aldosteron-system antagonists for treating renal insufficiency
InactiveUS20060286105A1Loss of renal functionPrevent and reduce riskBiocidePeptide/protein ingredientsRenal disorderFibrosis
The disclosure provides methods for treating, preventing, and reducing risk of occurrence of renal insufficiency in mammals. The disclosed methods include administering to a subject susceptible to, or afflicted with, renal disorder therapeutically effective amounts of a TGF-β antagonist and a renin-angiotensin-aldosterone system (RAAS) antagonist so as to maintain desirable levels of renal function. The populations treated by the methods of the invention include but are not limited to patients suffering or at risk for the development of renal insufficiency, such as those afflicted with type I or type II diabetes, hypertension, (auto)immune disease, systemic fibrosis, etc.
Owner:LEDBETTER STEVEN +2
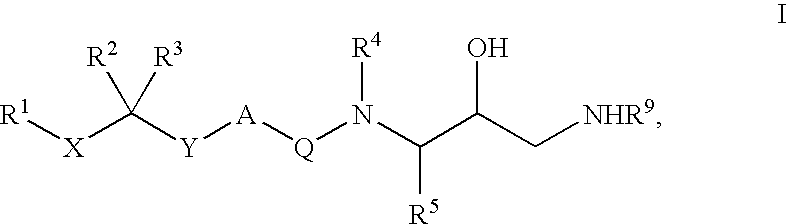
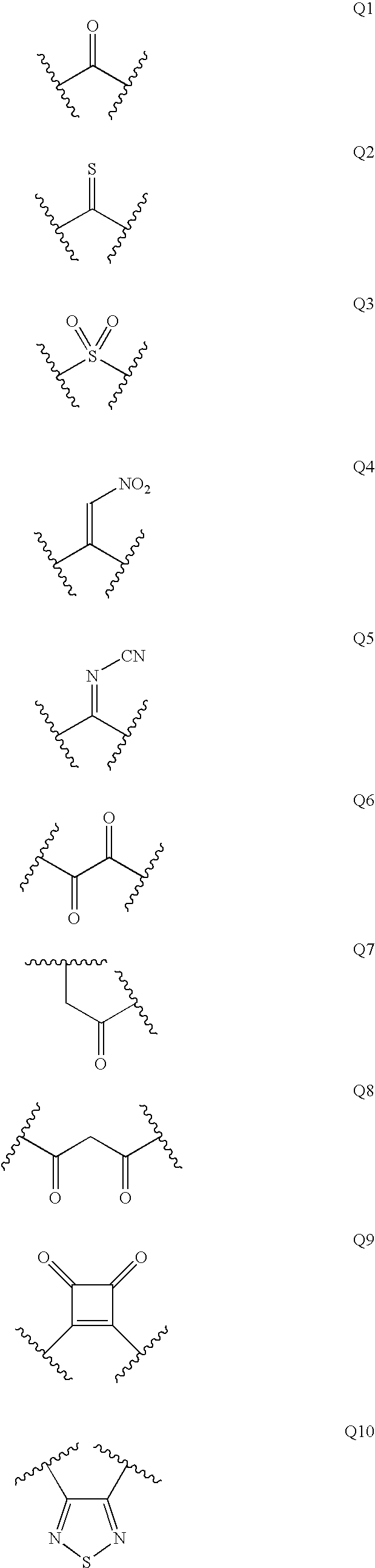
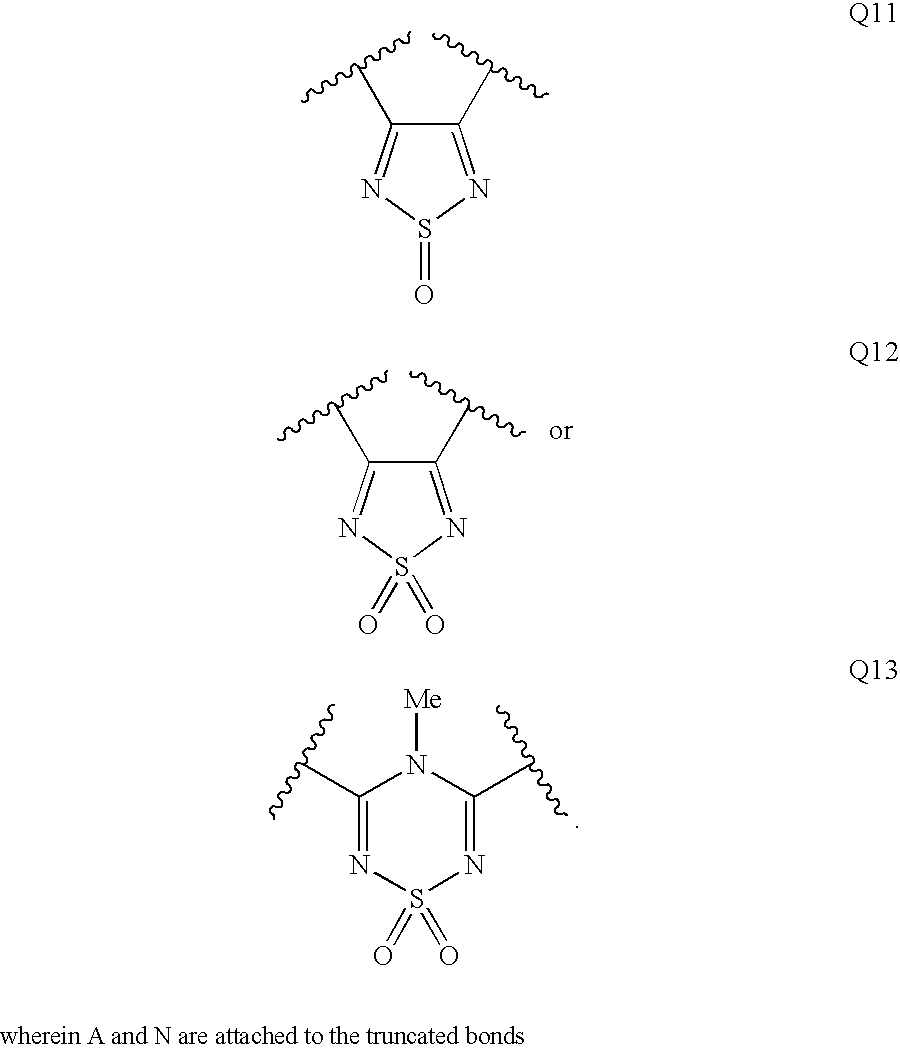
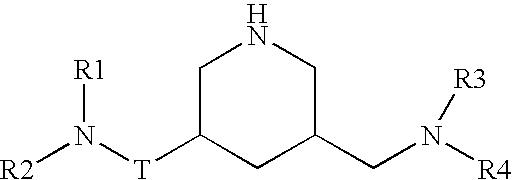
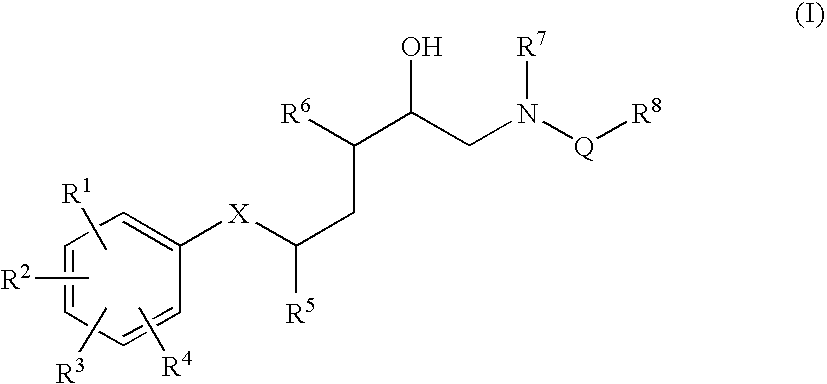
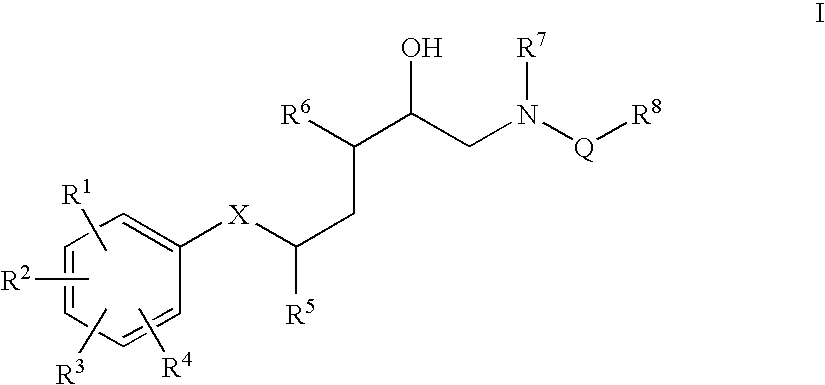
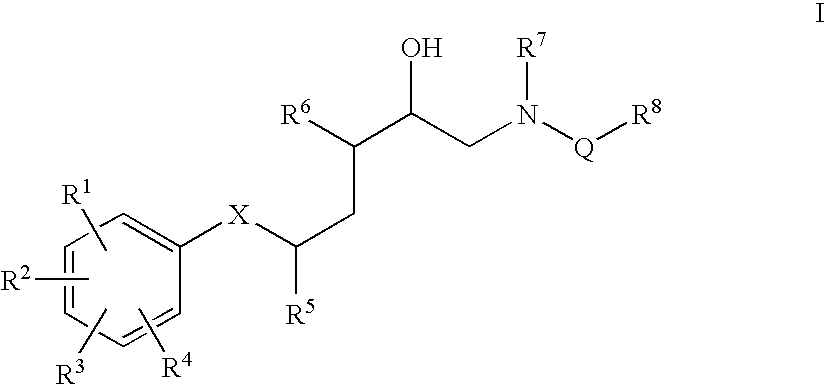
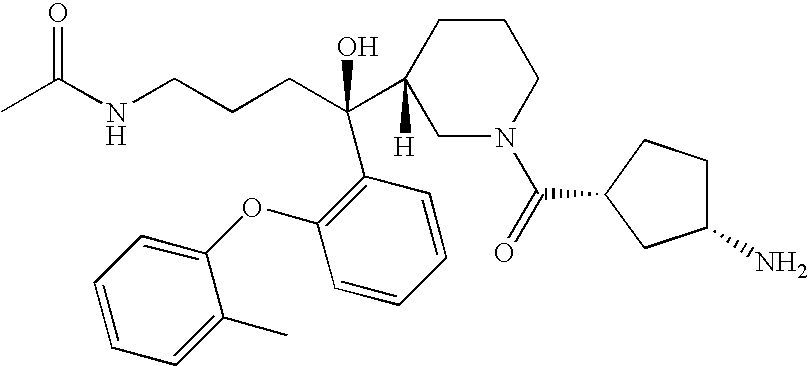
Diaminopropanol Renin Inhibitors
Described are diaminopropanols of which are orally active and bind to renin to inhibit its activity. They are useful in the treatment or amelioration of diseases associated with elevated levels of renin activity or in the treatment of aspartic protease mediated disorders. Also described is a method for the use of the diaminopropanols in ameliorating or treating renin related disorders in a subject in need thereof.
Owner:VITAE PHARMA INC
Angiotensin peptide-carrier conjugates and uses thereof
InactiveUS20060078554A1Promote antibody formationPromote formationVirusesHydrolasesVirus-like particleAngiotensinogenase
The present invention provides conjugates of peptide derivatives of the mammalian peptide hormones angiotensinogen, angiotensin I and angiotensin II, presented in a repetitive scaffold by coupling the peptide derivatives to a carrier, particularly a virus-like particle (VLP). The invention also provides methods of producing such conjugates, and immunotherapeutic uses of the resulting immunogen conjugates for the therapy and prophylaxis of conditions associated with the renin-activated angiotensin system.
Owner:CYTOS BIOTECHNOLOGY AG
Amide compounds and use of the same
InactiveUS20100324010A1Superior renin inhibitory activityHigh activityBiocideOrganic chemistryOrgan damageMedicinal chemistry
A renin inhibitor comprising a compound represented by the formula:wherein each symbol is as defined in the description, or a salt thereof or a prodrug thereof. The compound of the present invention has a superior renin inhibitory activity, and thus is useful as an agent for the prophylaxis or treatment of hypertension, various organ damages attributable to hypertension and the like.
Owner:TAKEDA PHARMA CO LTD
Renin inhibitors
InactiveUS20100160424A1Low costHigh activityBiocideCarbamic acid derivatives preparationDiseaseAspartic protease activity
Described are compounds that bind to aspartic proteases to inhibit their activity. They are useful in the treatment or amelioration of diseases associated with aspartic protease activity. Also described are methods of use of the compounds described herein in ameliorating or treating aspartic protease related disorders in a subject in need thereof.
Owner:VITAE PHARMA INC
Diaminopropanol renin inhibitors
Described are diaminopropanols of which are orally active and bind to renin to inhibit its activity. They are useful in the treatment or amelioration of diseases associated with elevated. levels of renin activity or in the treatment of aspartic protease mediated disorders. Also described is a method for the use of the diaminopropanols in ameliorating or treating renin related disorders in a subject in need thereof.
Owner:VITAE PHARMA INC
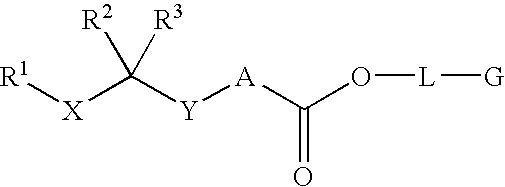
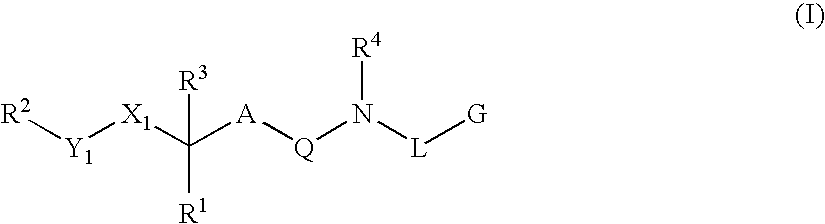
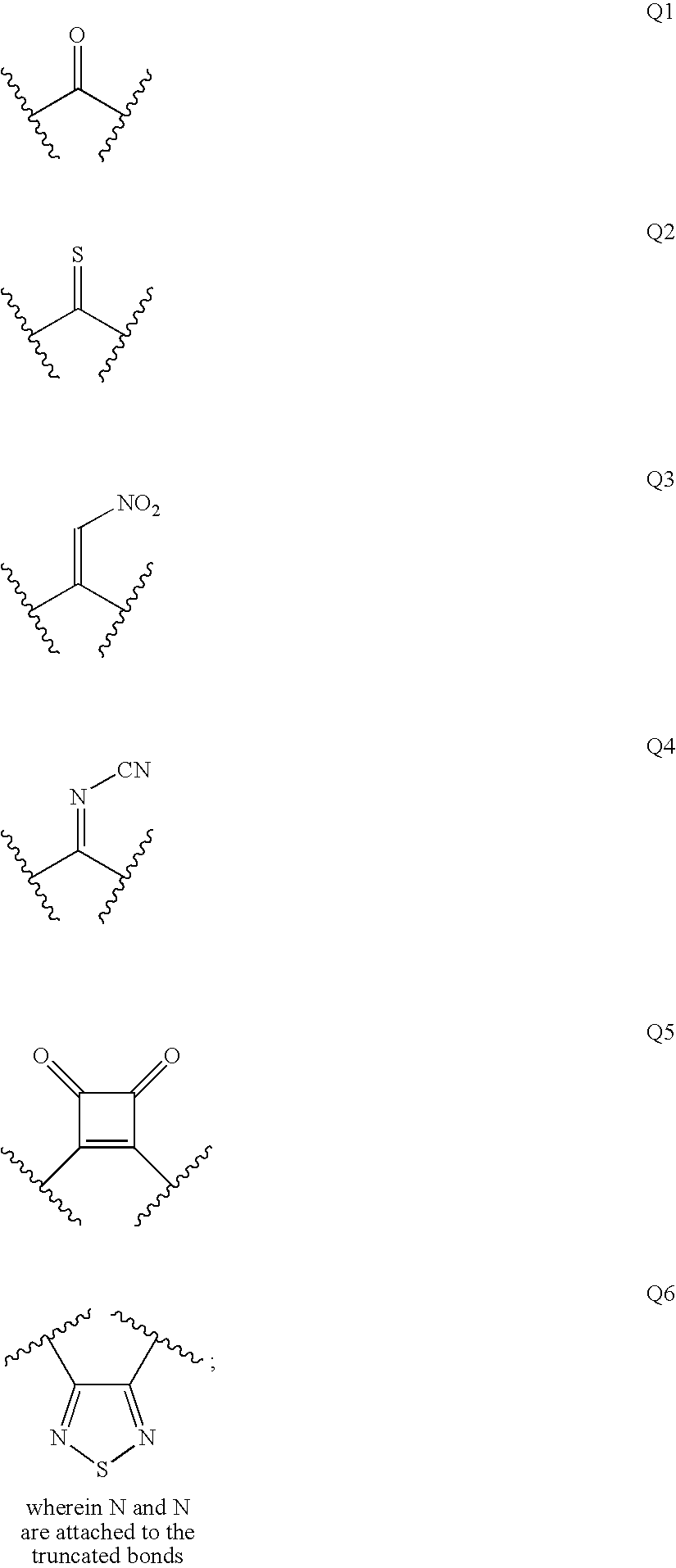
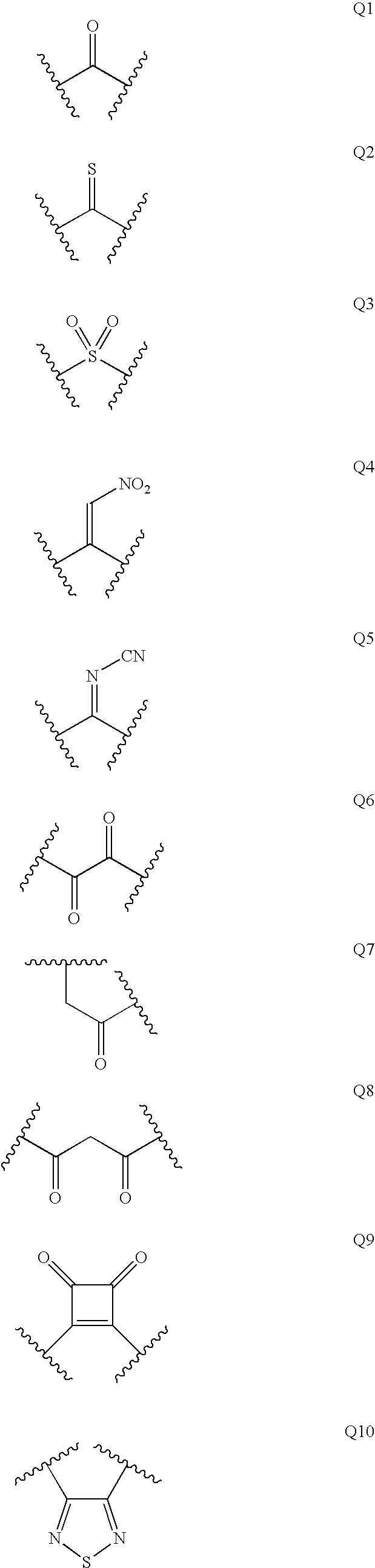
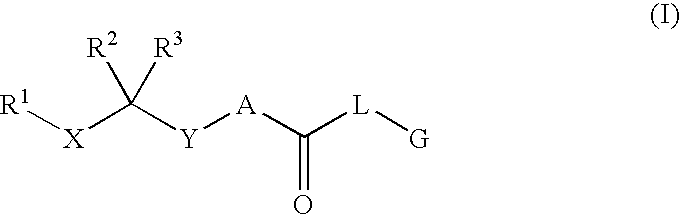
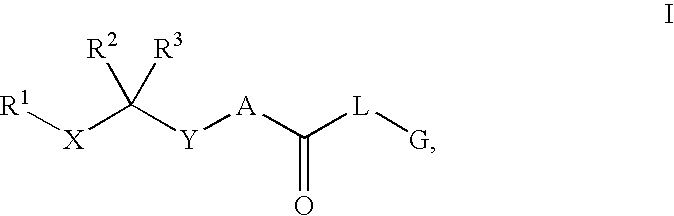
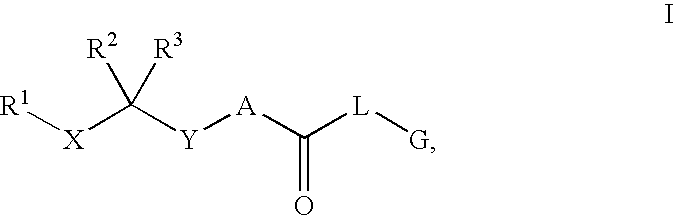
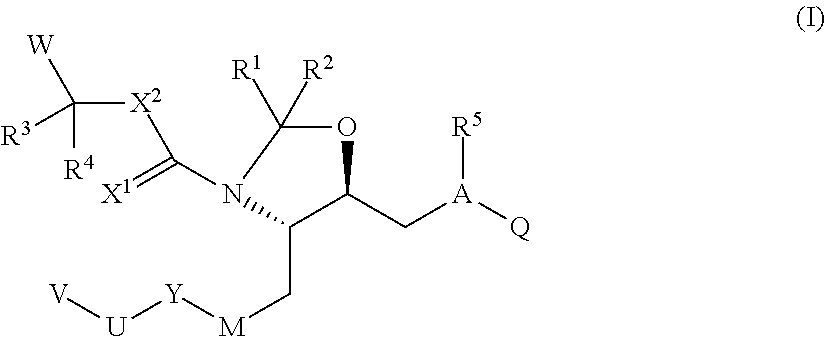
Renin Inhibitors
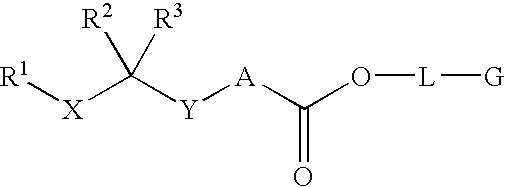
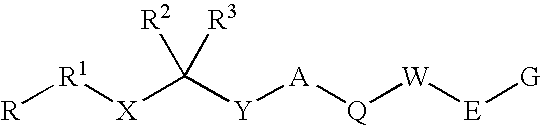
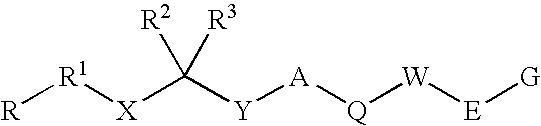
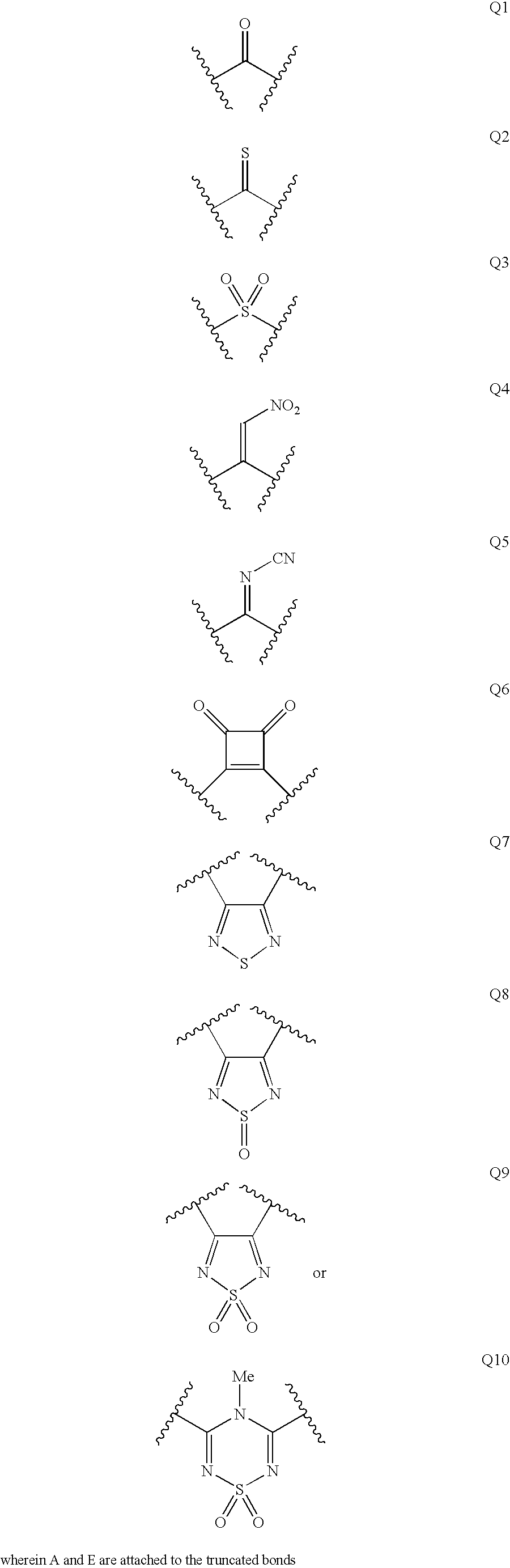
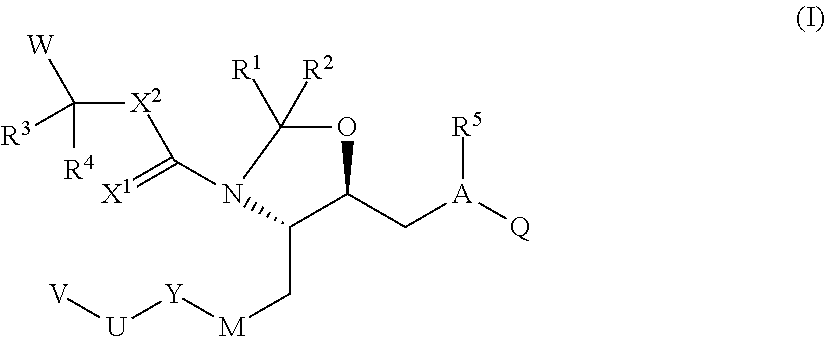
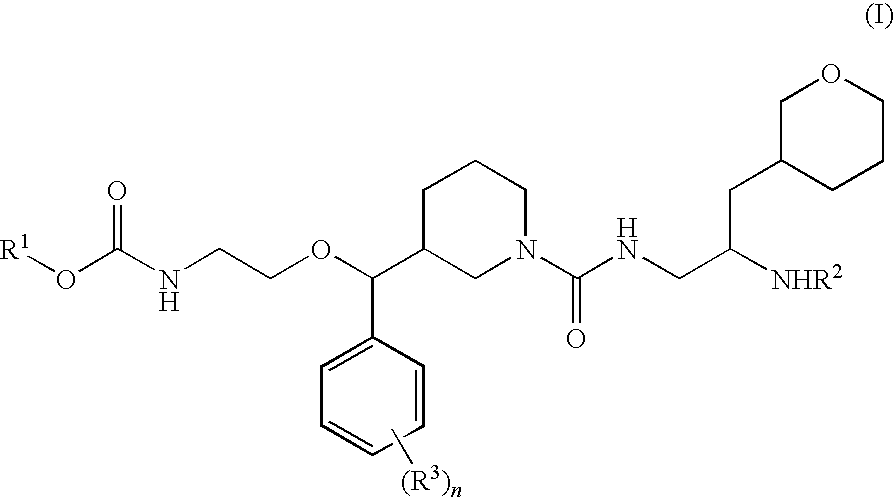
Disclosed are compounds having the formula (I): wherein the R1, R2, R3, X, Y, A, L, and G are defined herein. These compounds bind to aspartic proteases to inhibit their activity and are useful in the treatment or amelioration of diseases associated with aspartic protease activity. Also disclosed are methods of use of the compounds of Formula I for ameliorating or treating aspartic protease related disorders in a subject in need thereof.
Owner:VITAE PHARMA INC
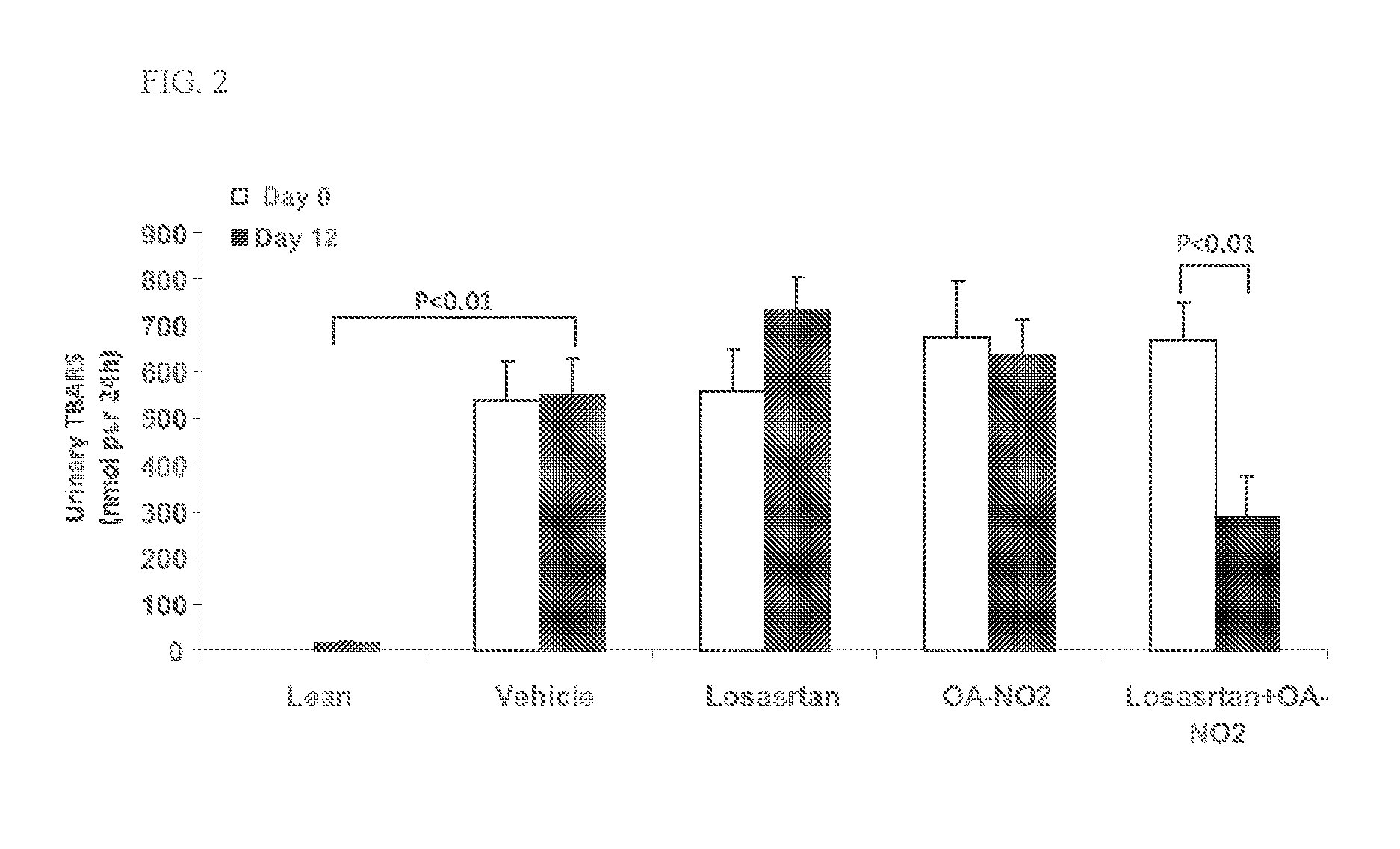
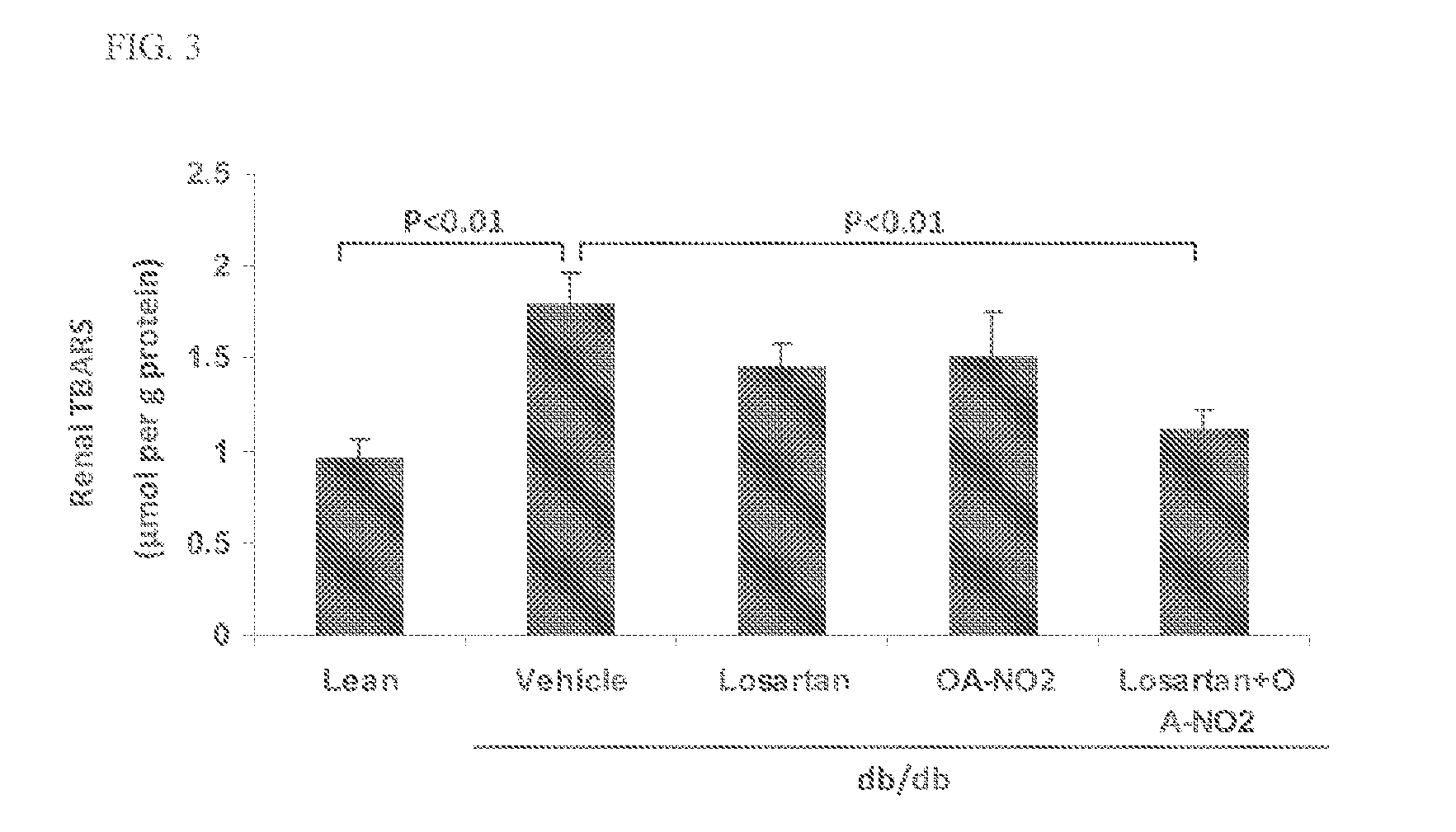
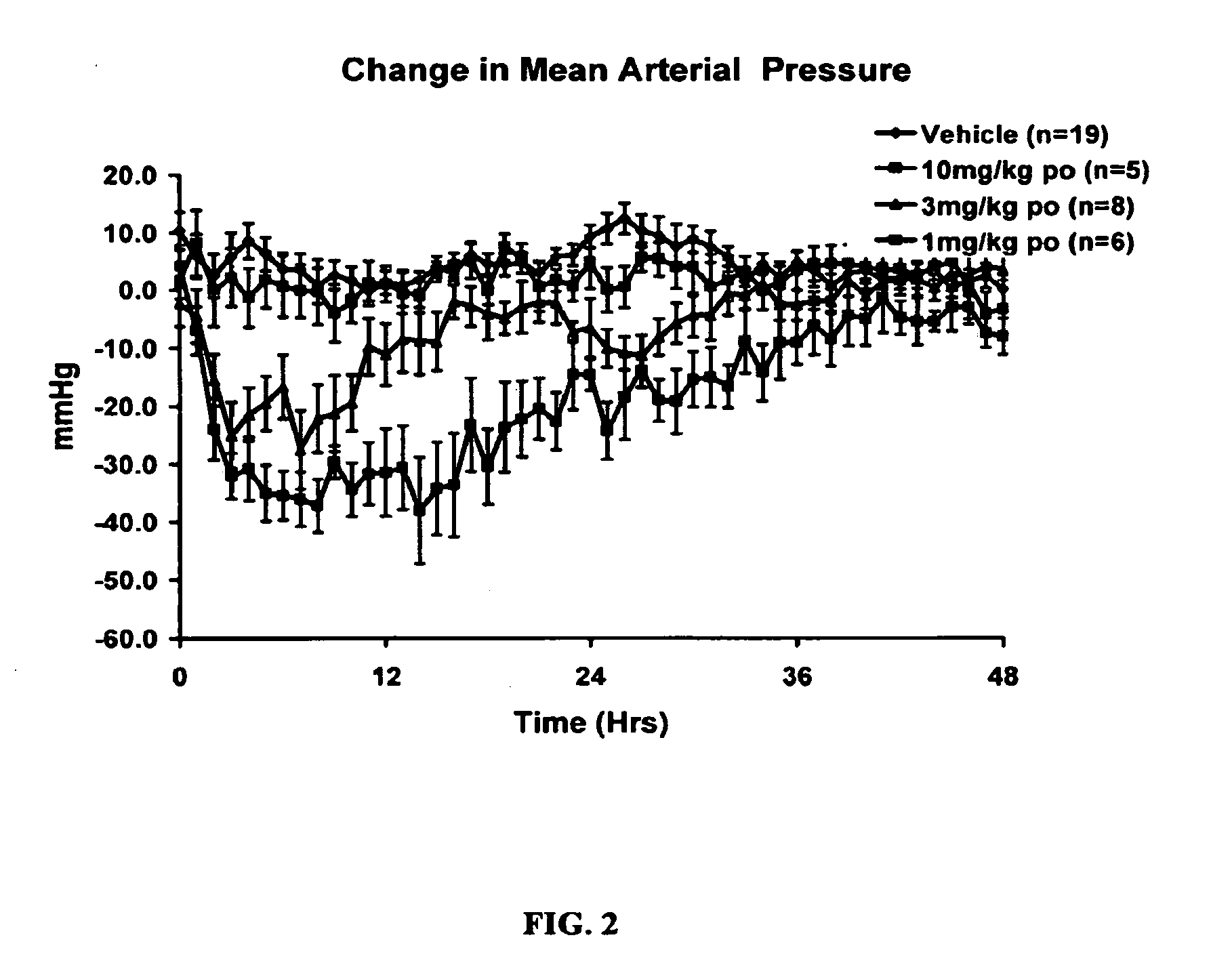
Combination therapy with nitrated lipids and inhibitors of the renin-angiotensin-aldosterone system
ActiveUS20140243380A1Reducing mRNA expressionHigh expressionBiocideUrinary disorderNephrosisEnd stage renal disease
The present technology provides compositions and methods for treating chronic kidney disease, end-stage renal disease, or diabetic nephropathy. The compositions comprise a nitrated lipid and an inhibitor of the renin-angiotensin-aldosterone system. The methods comprise administering a nitrated lipid in combination with an inhibitor of the renin-angiotensin-aldosterone system to a subject in need thereof, in an amount effective to treat diabetic nephropathy, chronic kidney disease, and / or end-stage renal disease. The use of a nitrated lipid with an inhibitor of the renin-angiotensin-aldosterone system exhibits a synergistic effect in treating chronic kidney disease and diabetic nephropathy.
Owner:UNIV OF UTAH RES FOUND
Renin Inhibitors
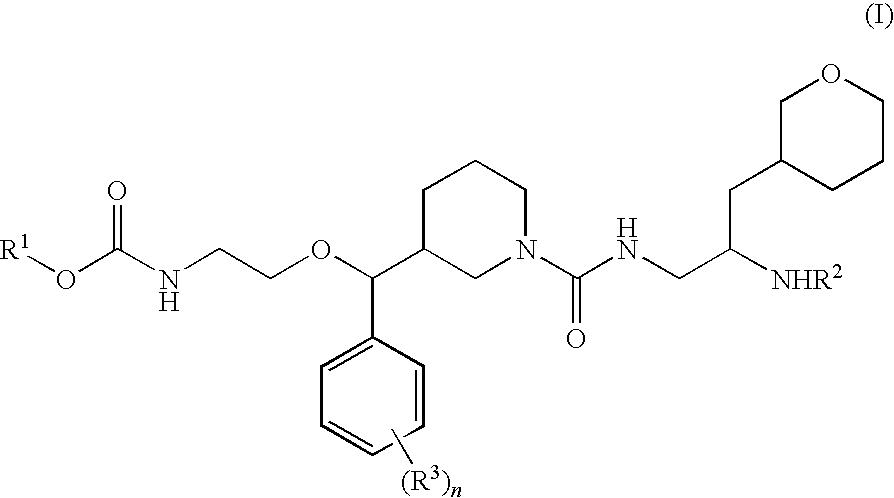
The present invention is directed to aspartic protease inhibitors represented by the following structural formula (I), or a pharmaceutically acceptable salt thereof. The present invention is also directed to pharmaceutical compositions comprising the aspartic protease inhibitors of Structural Formula (I). Methods of antagonizing one or more aspartic proteases in a subject in need thereof, and methods for treating an aspartic protease mediated disorder in a subject using these aspartic protease inhibitors are also disclosed.
Owner:VITAE PHARMA INC
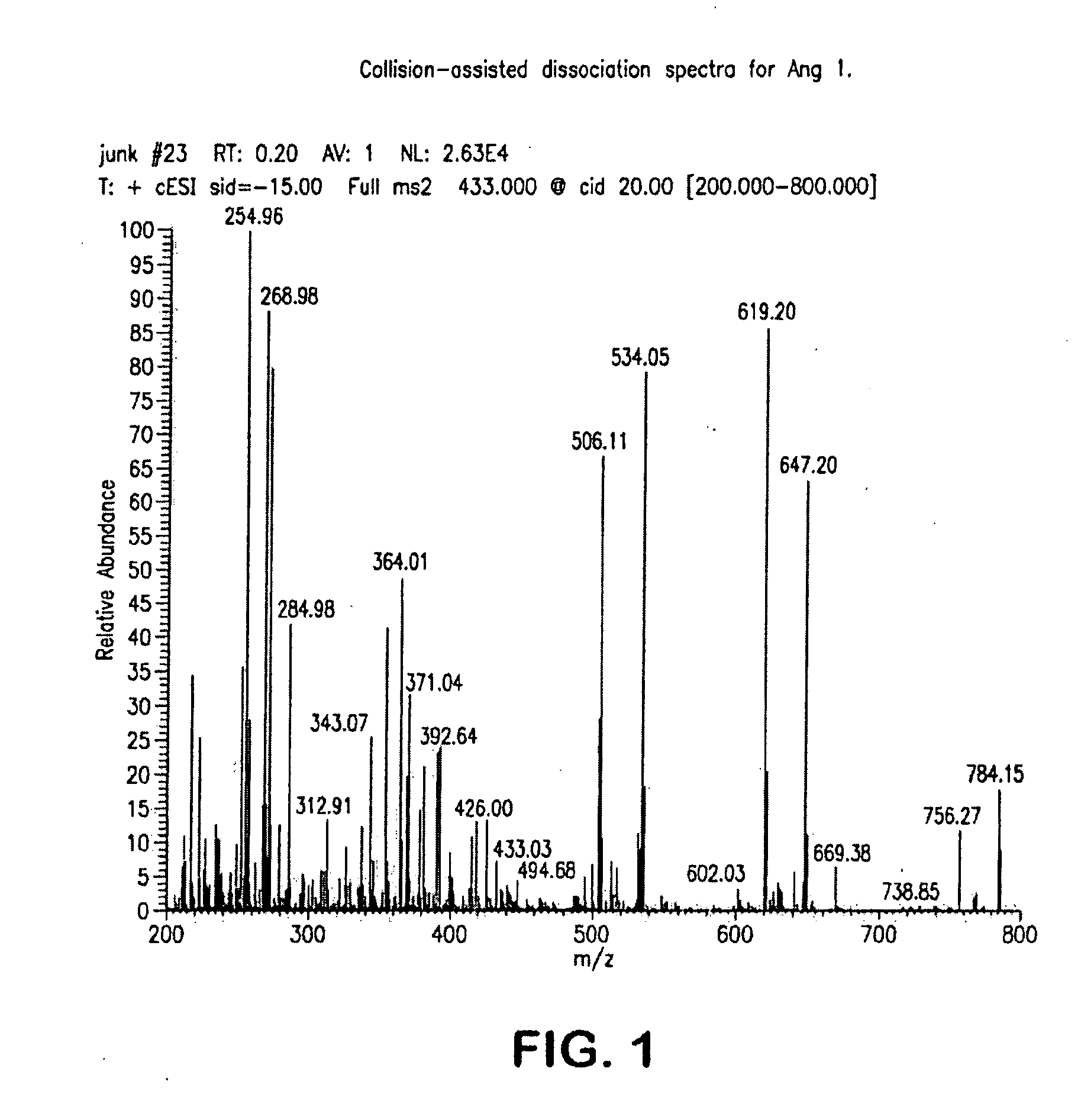
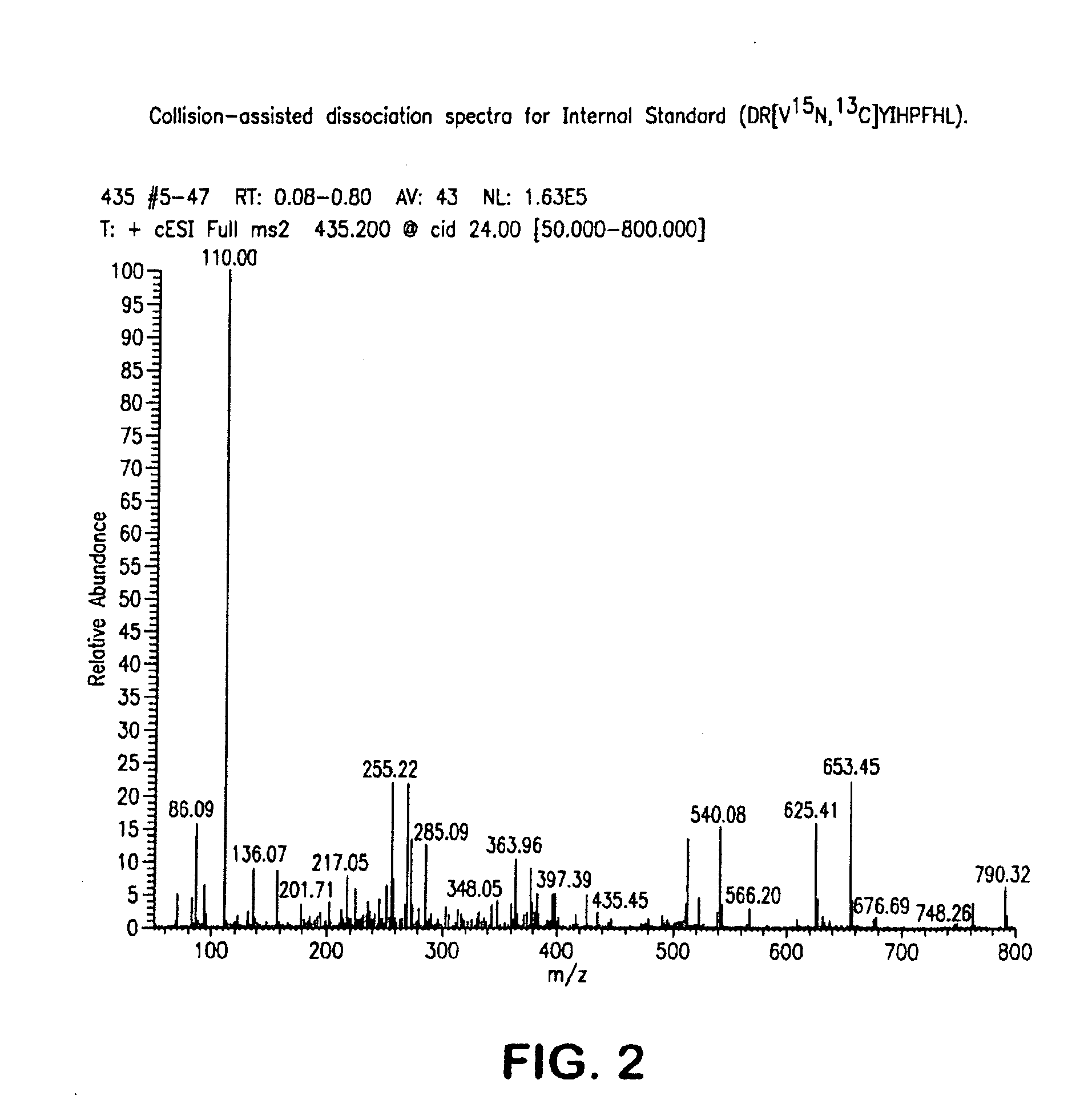
Mass spectrometry assay for plasma-renin
ActiveUS20100219338A1Avoid condensationSolvent is evaporatedIsotope separationMass spectrometersPlasma samplesMass Spectrometry-Mass Spectrometry
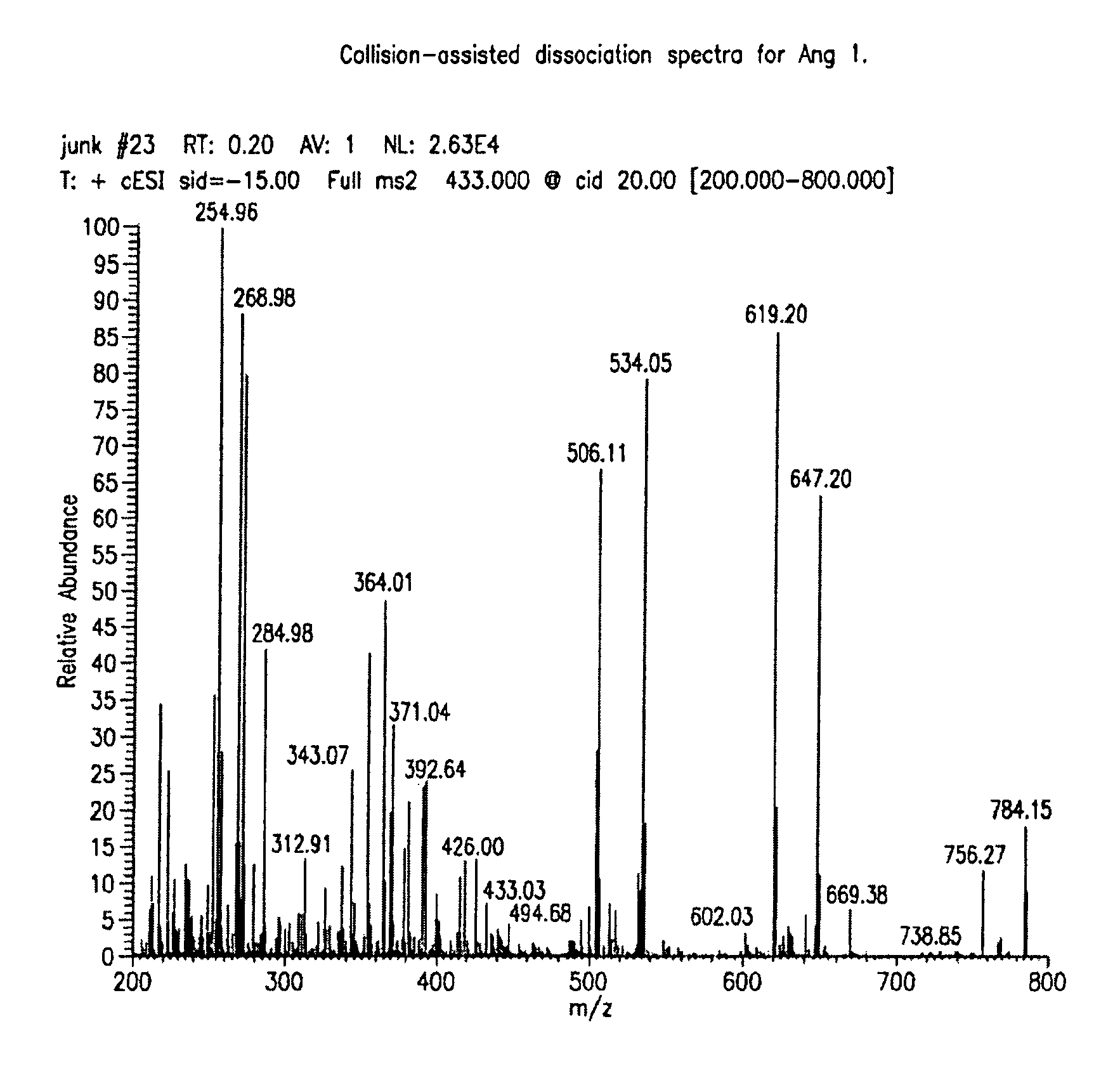
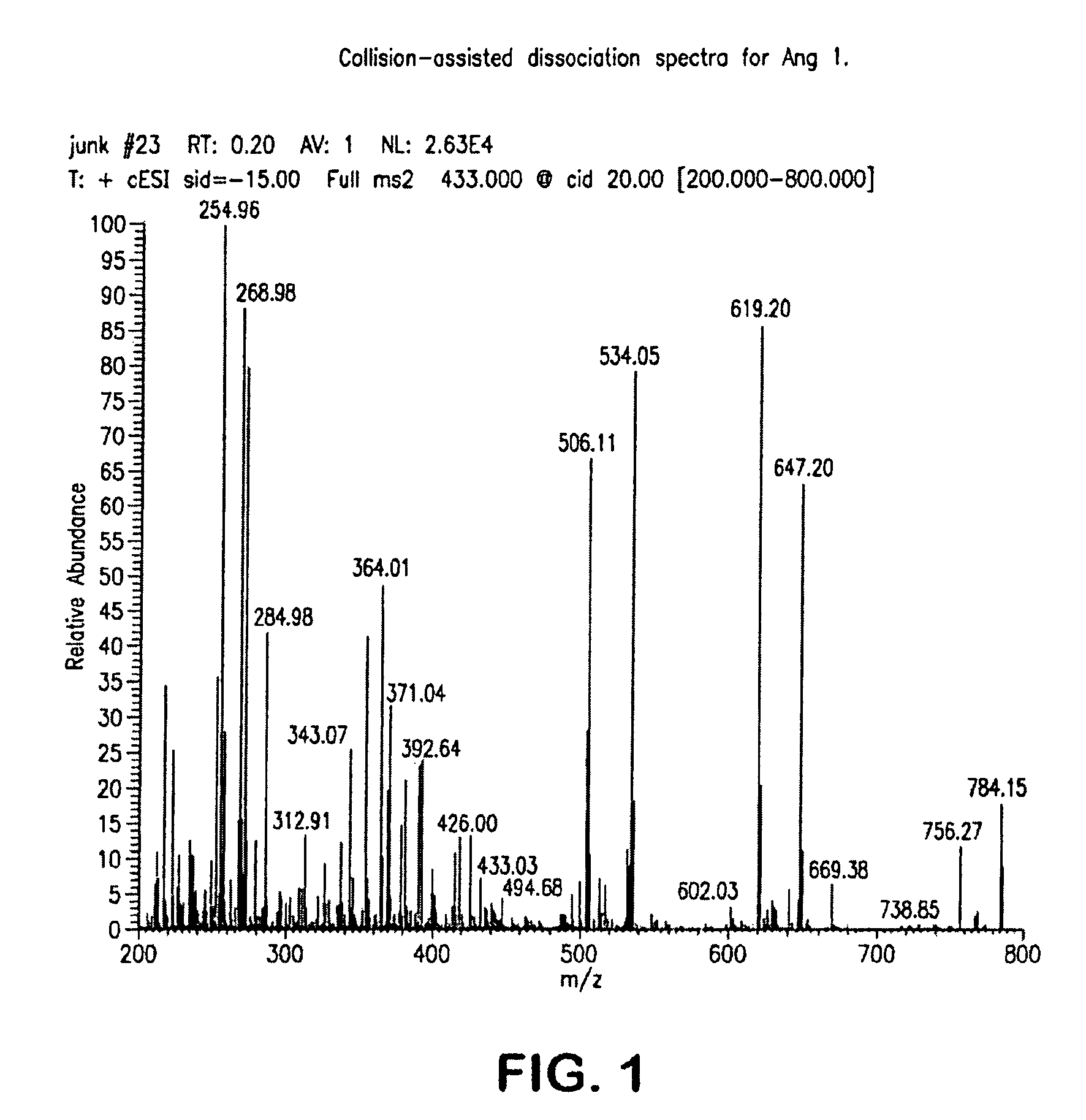
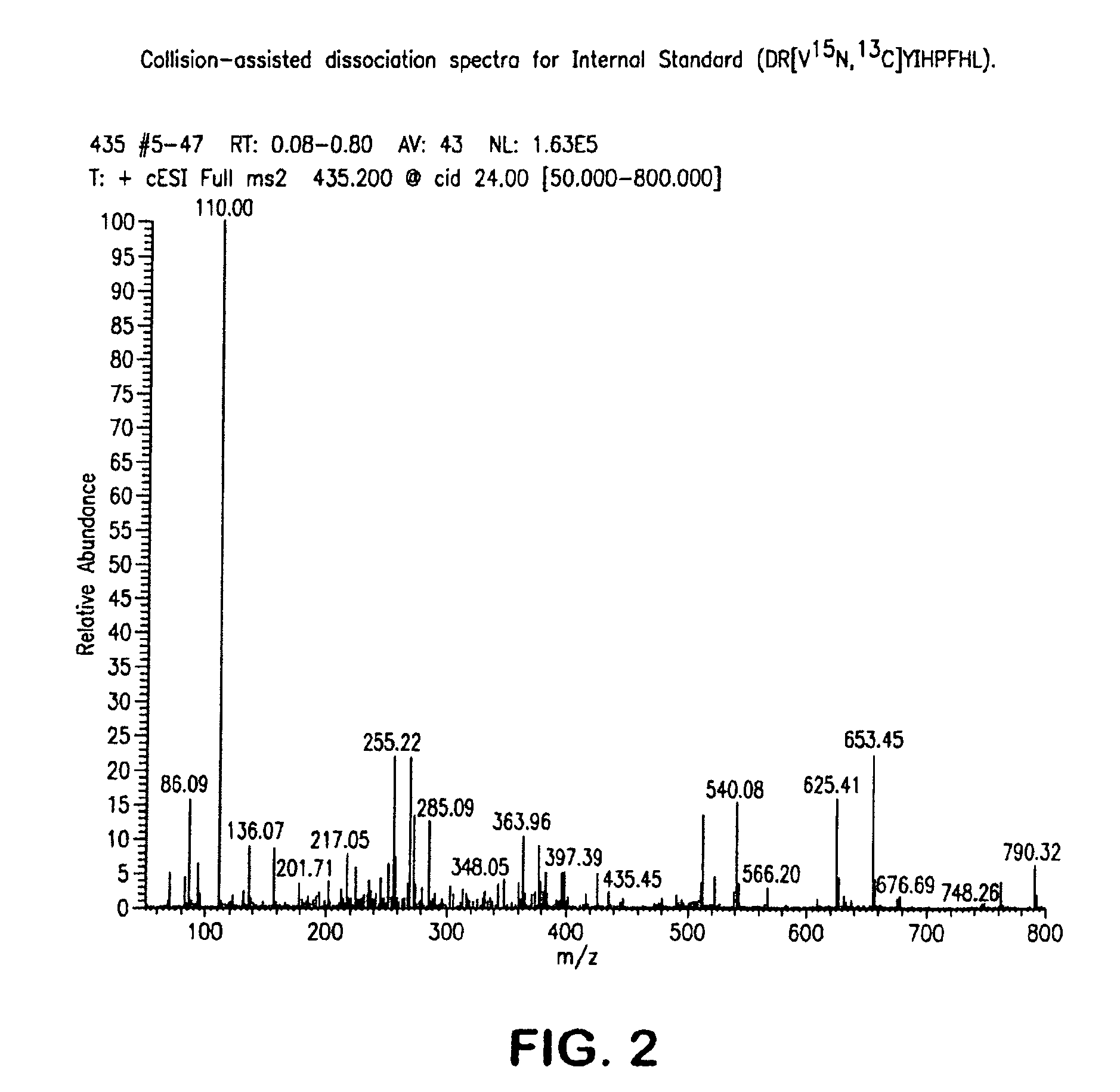
Provided are methods for measuring renin activity in a plasma sample using mass spectrometry. The methods generally involve ionizing purified angiotensin 1 from the sample and detecting the amount of angiotensin 1 ions generated. The amount of detected angiotensin 1 ions are then related to the amount of angiotensin 1 generated in the sample, which in turn is related to renin activity in the sample.
Owner:QUEST DIAGNOSTICS INVESTMENTS INC
Mass Spectrometry Assay for Plasma-Renin
ActiveUS20100032558A1Avoid condensationSolvent is evaporatedParticle separator tubesBiological material analysisPlasma samplesMass Spectrometry-Mass Spectrometry
Provided are methods for measuring renin activity in a plasma sample using mass spectrometry. The methods generally involve ionizing purified angiotensin 1 from the sample and detecting the amount of angiotensin 1 ions generated. The amount of detected angiotensin 1 ions are then related to the amount of angiotensin 1 generated in the sample, which in turn is related to renin activity in the sample.
Owner:QUEST DIAGNOSTICS INVESTMENTS INC
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
InactiveUS20120231476A1Eliminate needEasy to adaptDisease diagnosisBiological testingCathepsin BDipeptidyl peptidase
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using a one or more assays configured to detect a kidney injury marker selected from the group consisting of Cathepsin B, Renin, Dipeptidyl Peptidase IV, Neprilysin, Beta-2-microglobulin, Carbonic anhydrase IX, and C-X-C motif chemokine 2 as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
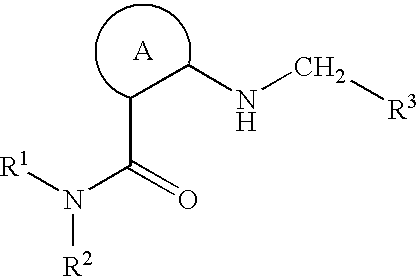
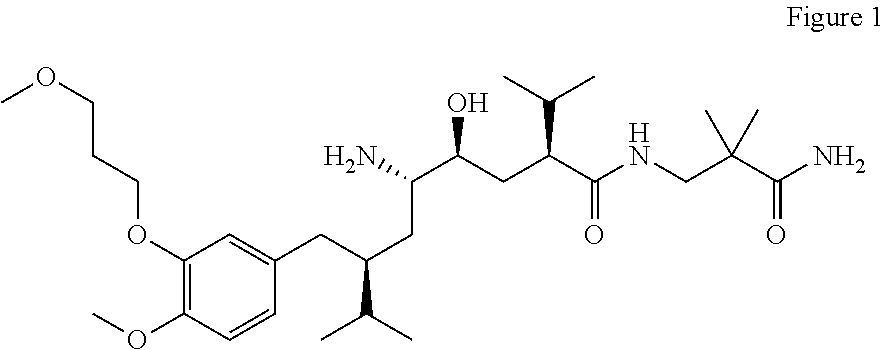
1-Acylamino-2-Hydroxy-3-Amino- -Arylalkanes as Renin Inhibitors
Owner:VITAE PHARMA INC
Mass spectrometry assay for plasma-renin
ActiveUS7834313B2Facilitate desorptionGood removal effectBiological material analysisMaterial analysis by optical meansMass Spectrometry-Mass SpectrometryBlood plasma
Provided are methods for measuring renin activity in a plasma sample using mass spectrometry. The methods generally involve ionizing purified angiotensin 1 from the sample and detecting the amount of angiotensin 1 ions generated. The amount of detected angiotensin 1 ions are then related to the amount of angiotensin 1 generated in the sample, which in turn is related to renin activity in the sample.
Owner:QUEST DIAGNOSTICS INVESTMENTS INC
Compositions and Methods for Treatment of Renin-Angiotensin Aldosterone System (RAAS)- Related Disorders
InactiveUS20110257130A1Reducing ACE activityReduced activityBiocideNervous disorderAngiotensin-converting enzymeDisease
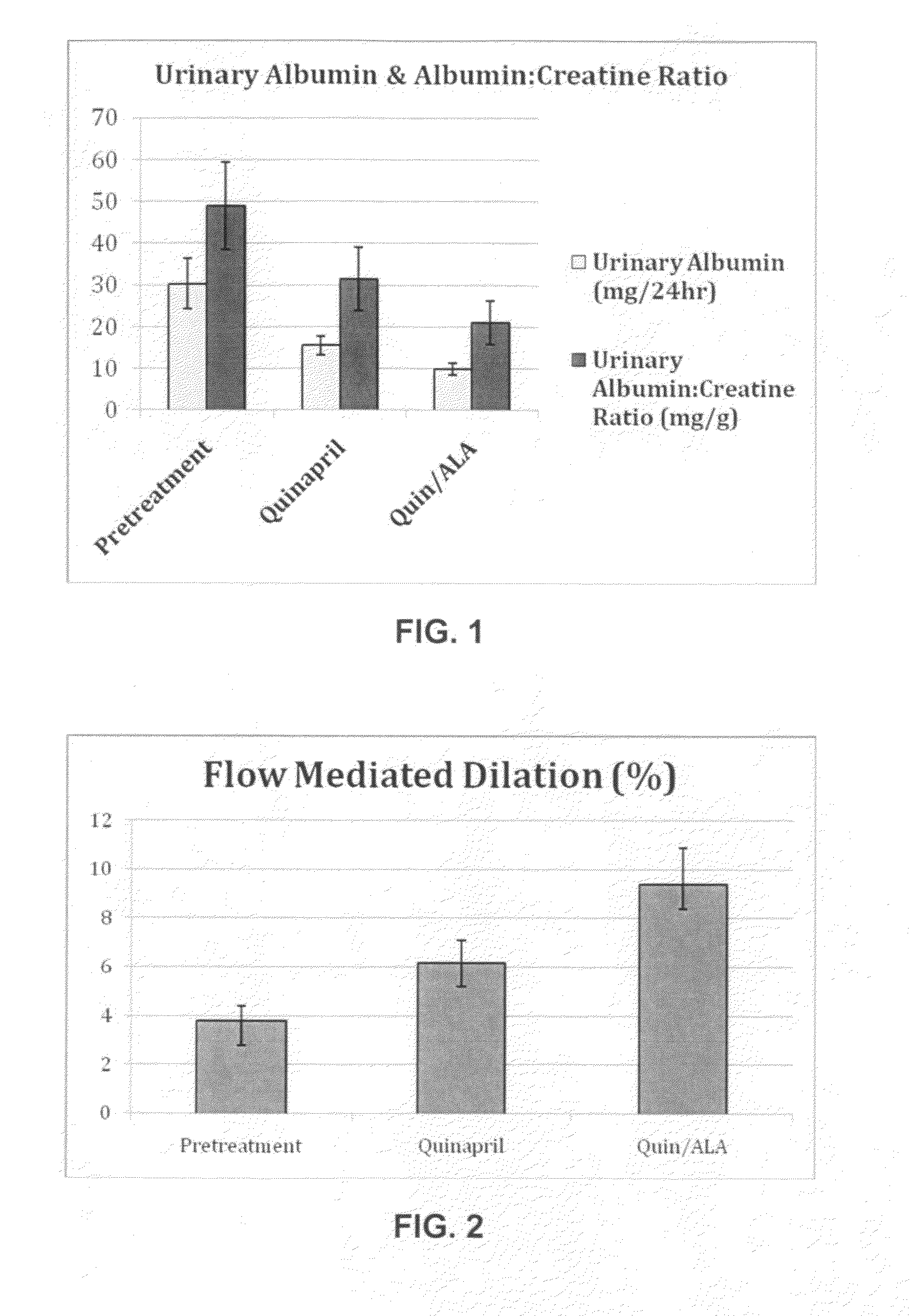
Compounds are provided which can be useful in reducing the activity of an angiotensin-converting enzyme and thus be used to treat or prevent a renin-angiotensin aldosterone system-related disorder. These compounds include lipoic acid derivatives such as prolyl lipoic acid and pipecolinyl lipoic acid, and other compounds, and these compounds are useful in treating hypertension, stroke, or other renin-angiotensin aldosterone system-related disorders in human or animal patients. Pharmaceutical compositions prepared using these compounds and methods of treatment using these compounds are also provided.
Owner:CARMEL BIOSCI
Treatment of Muscular Conditions and Muscular Dystrophies
InactiveUS20140256644A1Reduced mechanical propertiesDecreasing cytoskeletal stiffnessBiocidePeptide/protein ingredientsDiseaseMuscular dystrophy
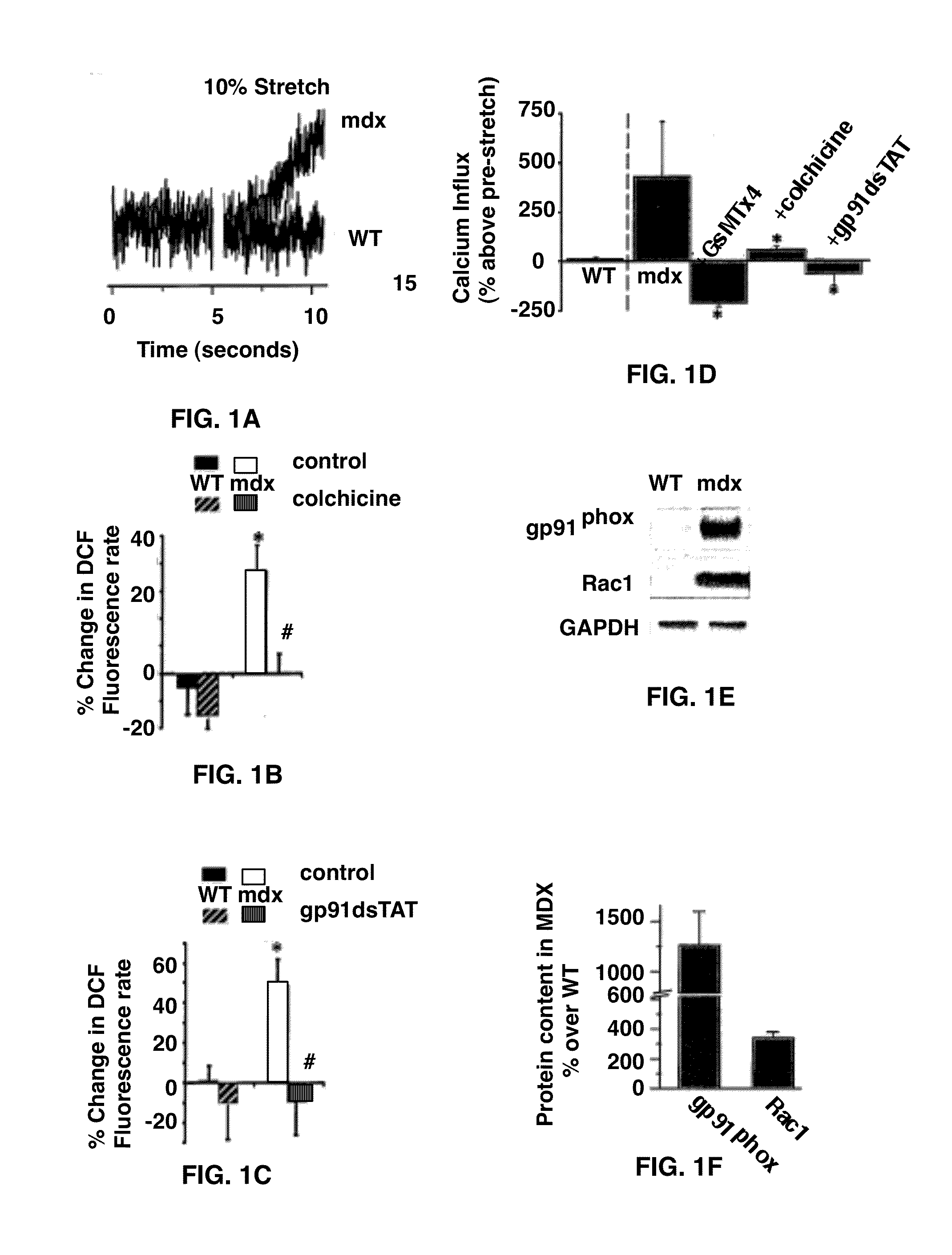
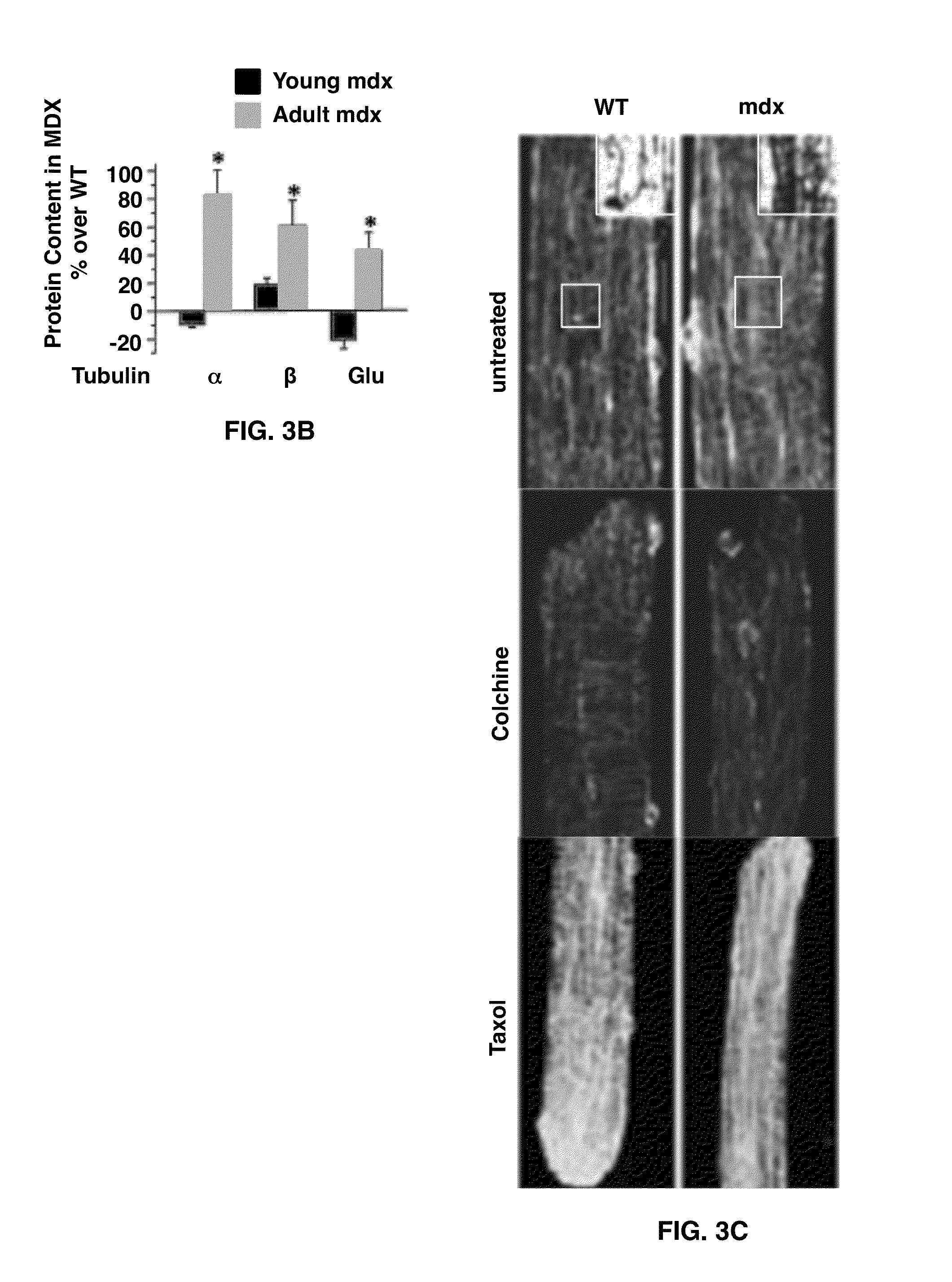
The present invention provides methods for improving muscular function or treating a muscular disorder in an individual by administering to the individual a pharmacologically effective amount of a compound that inhibits microtubule-dependent NADPH Oxidase 2 reactive oxygen species signaling production. In addition compounds that block sarcolemmal Ca2+ channel activation and / or renin-angiotensin signaling may be administered with the inhibitor of microtubule-dependent NADPH Oxidase 2 reactive oxygen species signaling production.
Owner:UNIV OF MARYLAND BALTIMORE
Renin inhibitors
InactiveUS20090275581A1Low costHigh activityBiocideOrganic chemistryDiseaseAspartic protease activity
Disclosed are compounds according to Formula I:wherein the variables are defined herein. Such compounds are can bind aspartic proteases to inhibit their activity. They are useful in the treatment or amelioration of diseases associated with aspartic protease activity.Also described herein are methods of antagonizing aspartic protease inhibitors in a subject in need thereof comprising administering to the subject a therapeutically effective amount of a compound according to Formula I.
Owner:VITAE PHARMA INC
Antihypertensive rapeseed peptide and preparation method and application of antihypertensive rapeseed peptide
ActiveCN102676621AWide variety of sourcesImprove protectionHydrolysed protein ingredientsFermentationEnzymatic hydrolysisProteinase activity
The invention provides an antihypertensive rapeseed peptide and a preparation method and an application of the antihypertensive rapeseed peptide. The method for preparing the antihypertensive rapeseed peptide includes using protease to hydrolyze a rapeseed protein water solution, then performing refining to obtain the antihypertensive rapeseed peptide. When the method for preparing the antihypertensive rapeseed peptide is compared with methods for preparing the antihypertensive rapeseed peptide in prior art, rapeseed meals or rapeseed isolated proteins are directly used as raw materials to obtain the antihypertensive rapeseed peptide by means of enzymatic hydrolysis, the sources of raw materials are wide, the production process is simple, the cost is low, the waste can be changed into the valuable, and environment protection is facilitated. The antihypertensive rapeseed peptide can be used for not only inhibiting angiotensin converting enzyme (ACE) activity, but also inhibiting renin activity to block an alternative pathway of a renin angiotensin system (RAS), the alternative pathway of the RAS is activated due to the fact that hypotensor of ACE inhibitor is taken for a long time, and the antihypertensive rapeseed peptide has a great developing prospect in decreasing blood pressure.
Owner:NANJING UNIV OF FINANCE & ECONOMICS
Renin Inhibitors
Described are compounds which bind to aspartic proteases to inhibit their activity. They are useful in the treatment or amelioration of diseases associated with aspartic protease activity. Also described are methods of use of the compounds described herein in ameliorating or treating aspartic protease related disorders in a subject in need thereof.
Owner:VITAE PHARMA INC
Novel 1,3-oxazolidine compounds and their use as renin inhibitors
The present invention relates to certain novel 1,3-oxazolidine compounds of formula (I), to processes for making such compounds and to their utility as renin inhibitors or prodrugs of renin inhibitors.
Owner:MEDIVIR AB
(-)-Hydroxycitric acid for the modulation of angiotensin-converting enzyme
InactiveUS20060025482A1Preventing and treating and ameliorating conditionStabilize and improve healthBiocideAnimal repellantsDiagnostic Radiology ModalityAdjuvant
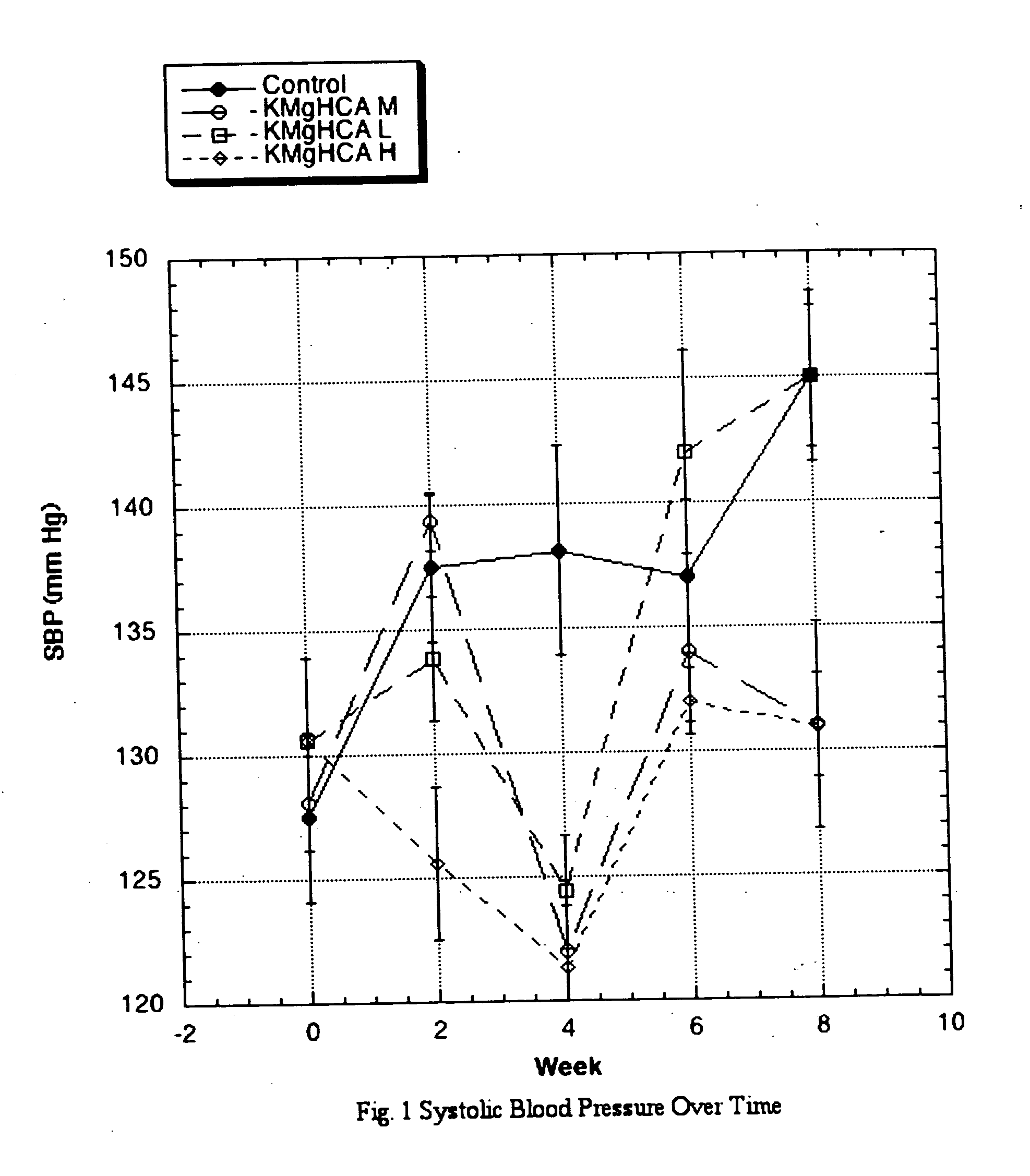
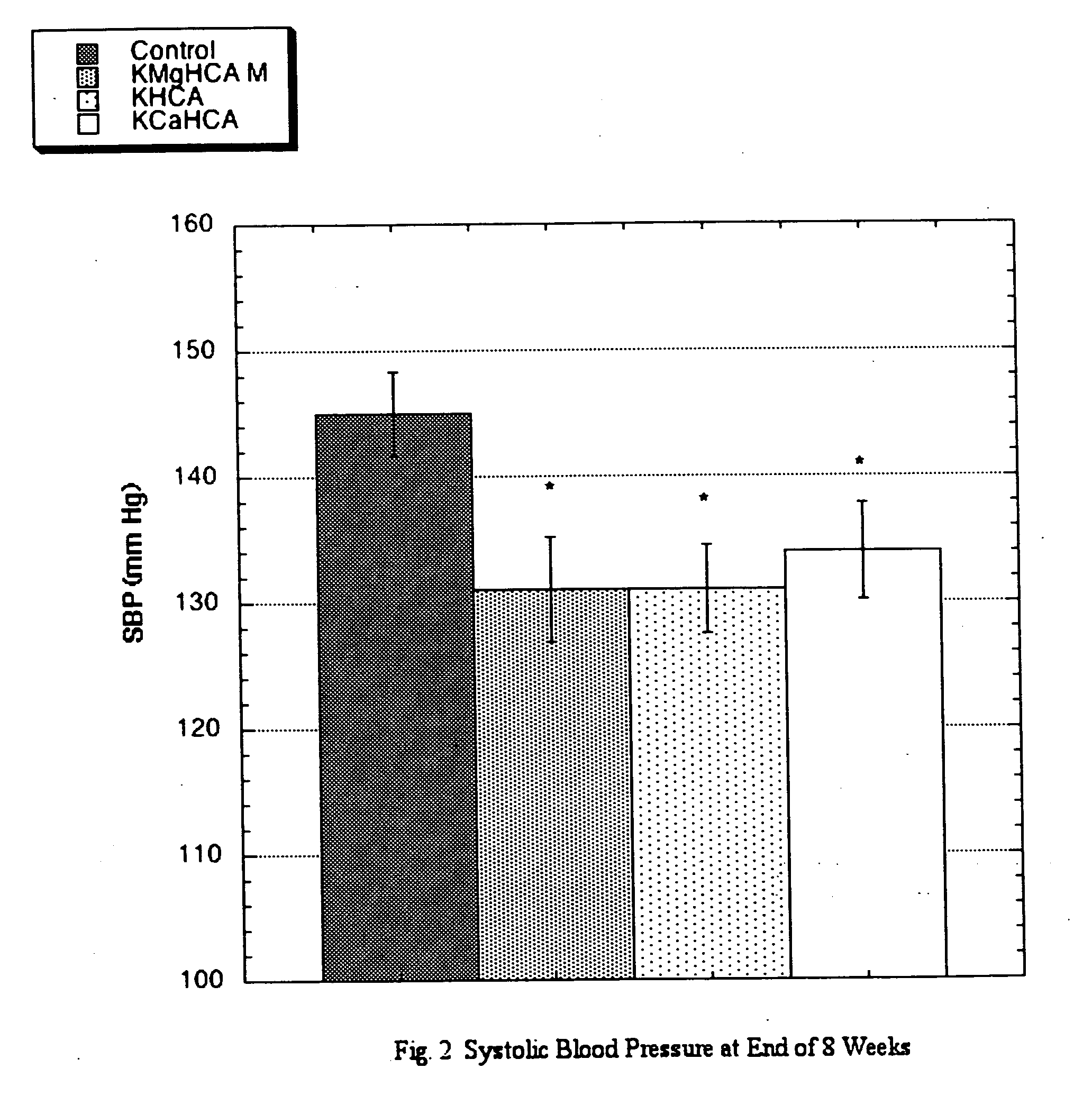
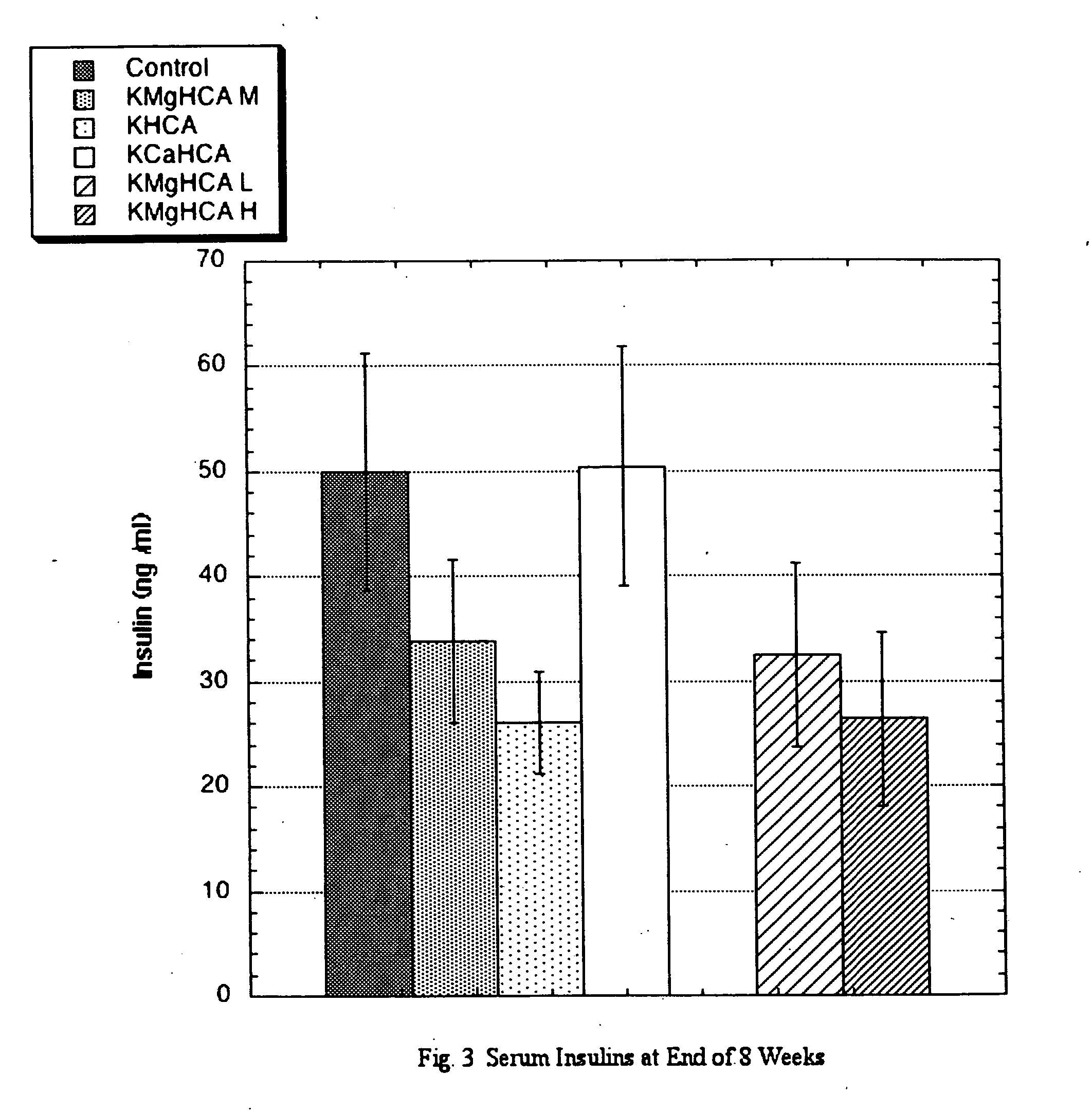
The invention teaches that supplementation with (−)-hydroxycitrate constitutes a novel means of modulating the angiotensin-converting enzyme (ACE) / renin-angiotensin-aldosterone system and is useful for preventing, treating and ameliorating conditions involving the angiotensin-converting enzyme (ACE) / renin-angiotensin-aldosterone system. The discovery that HCA has angiotensin-converting enzyme (ACE) / renin-angiotensin-aldosterone system-moderating effects allows for the creation of novel and more efficacious approaches to preventing and ameliorating conditions that arise from excessive ACE activity. These include cardiovascular diseases in general, heart failure, ventricular remodeling, ejection fraction issues, atrial fibrillation, and a wide variety of renal conditions. Other health conditions discovered to be influenced by the angiotensin-converting enzyme (ACE) / renin-angiotensin-aldosterone system would similarly be expected to be influenced. It is yet a further advantage of the present invention to provide a means—one that is accompanied by few or no side effects—of maintaining such improved status without resort to special diets. Furthermore, this discovery makes possible the development of adjuvant modalities that can be used to improve the results realized with other treatment compounds while at the same time reducing the side effects normally found with such drugs. HCA delivered in the form of its potassium salt is efficacious at a daily dosage (bid or tid) of between 750 mg and 10 grams, preferably at a dosage of between 3 and 6 grams for most individuals. A daily dosage above 10 grams might prove desirable under some circumstances, such as with extremely large or resistant individuals, but this level of intake is not deemed necessary under normal conditions.
Owner:CLOUATRE DALLAS L
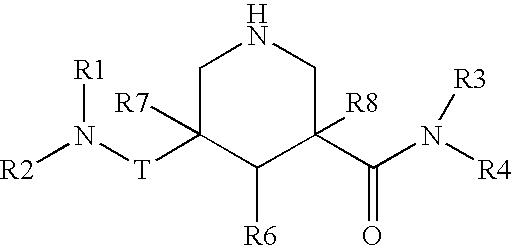
Piperidine renin inhibitors
The present invention is directed to aspartic protease inhibitors represented by the following structural formula (I), or a pharmaceutically acceptable salt thereof. The present invention is also directed to pharmaceutical compositions comprising the aspartic protease inhibitors of Structural Formula (I). Methods of antagonizing one or more aspartic proteases in a subject in need thereof, and methods for treating an aspartic protease mediated disorder in a subject using these aspartic protease inhibitors are also disclosed.
Owner:VITAE PHARMA INC
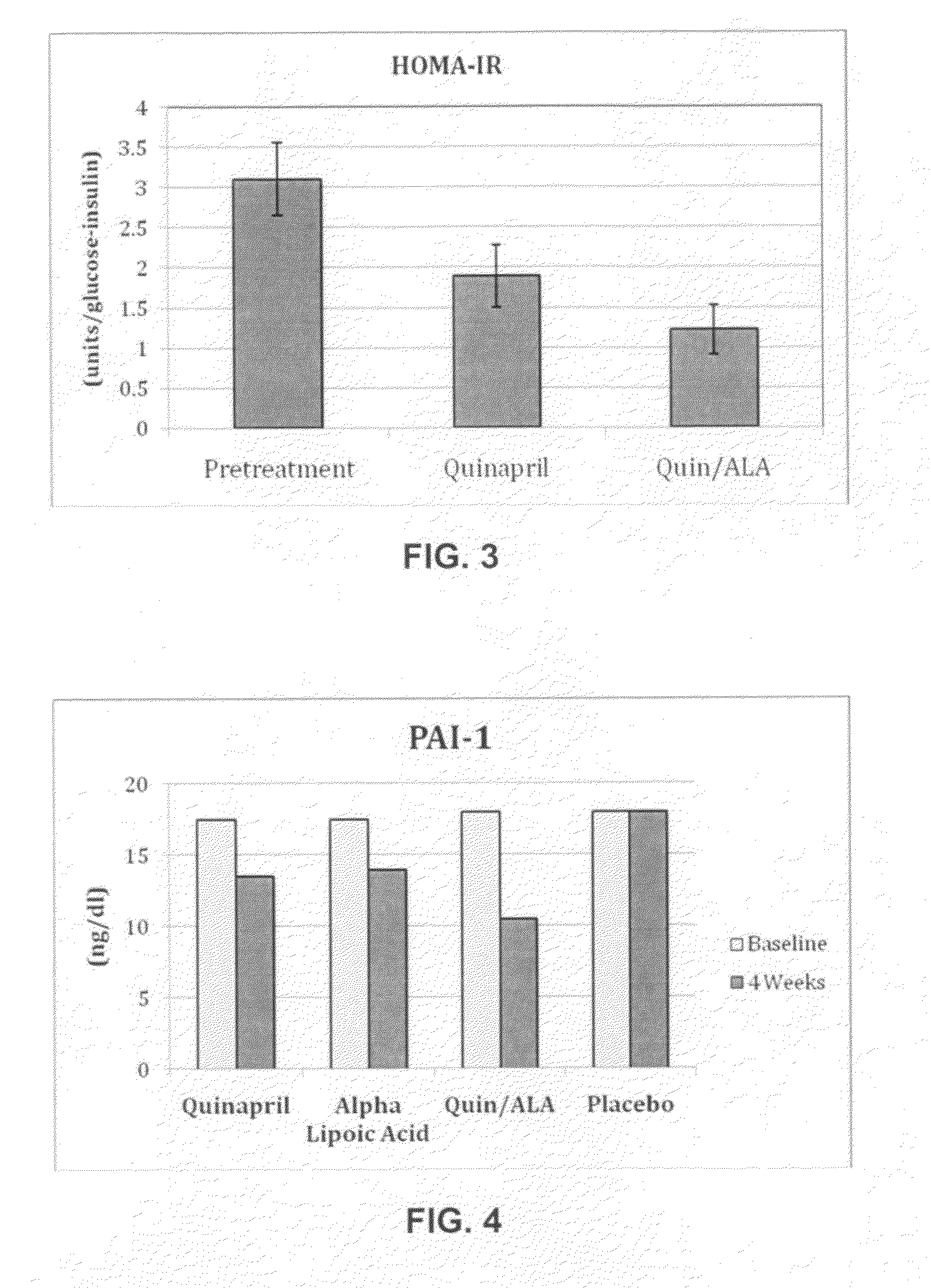
Compositions comprising renin-angiotensin aldosterone system inhibitors and lipoic acid compounds, and the use thereof for the treatment of renin-angiotensin aldosterone system-related disorders
InactiveUS20100173936A1Increase vasodilationReduce the amount requiredBiocideAntipyreticRisk strokeArterial Vasodilation
Compositions are provided which can be useful in treating a renin-angiotensin aldosterone system-related disorder. These compositions include renin-angiotensin aldosterone system inhibitors and lipoic acid compounds, as well as other therapeutic agents, and are useful in treating hypertension, stroke, metabolic syndrome, or other renin-angiotensin aldosterone system-related disorders in a subject. The compositions are also useful in improving vasodilation, reducing proteinuria, and reducing insulin resistance in a subject. Pharmaceutical compositions and methods of treatment using the compositions are further provided.
Owner:CARMEL BIOSCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com