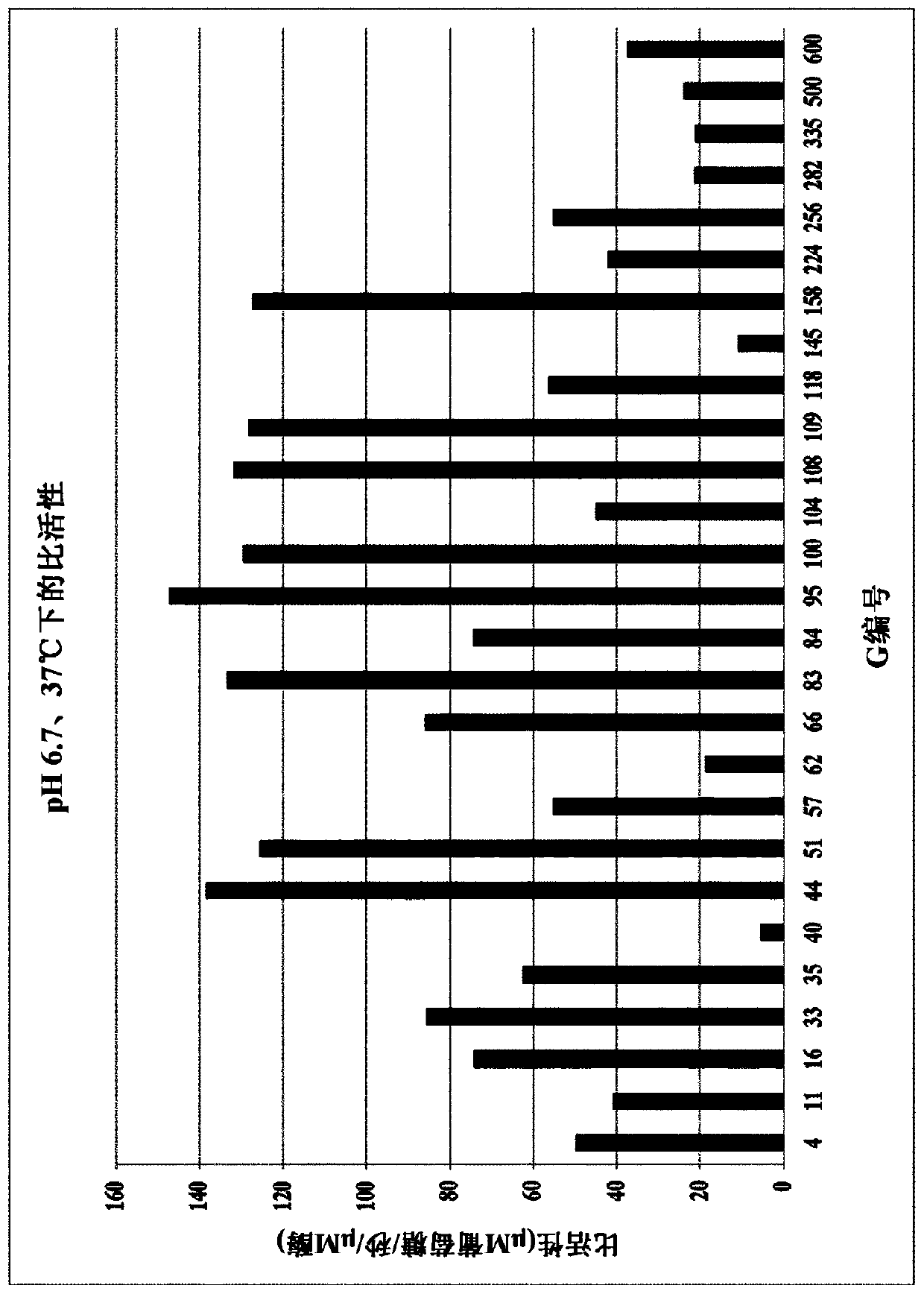
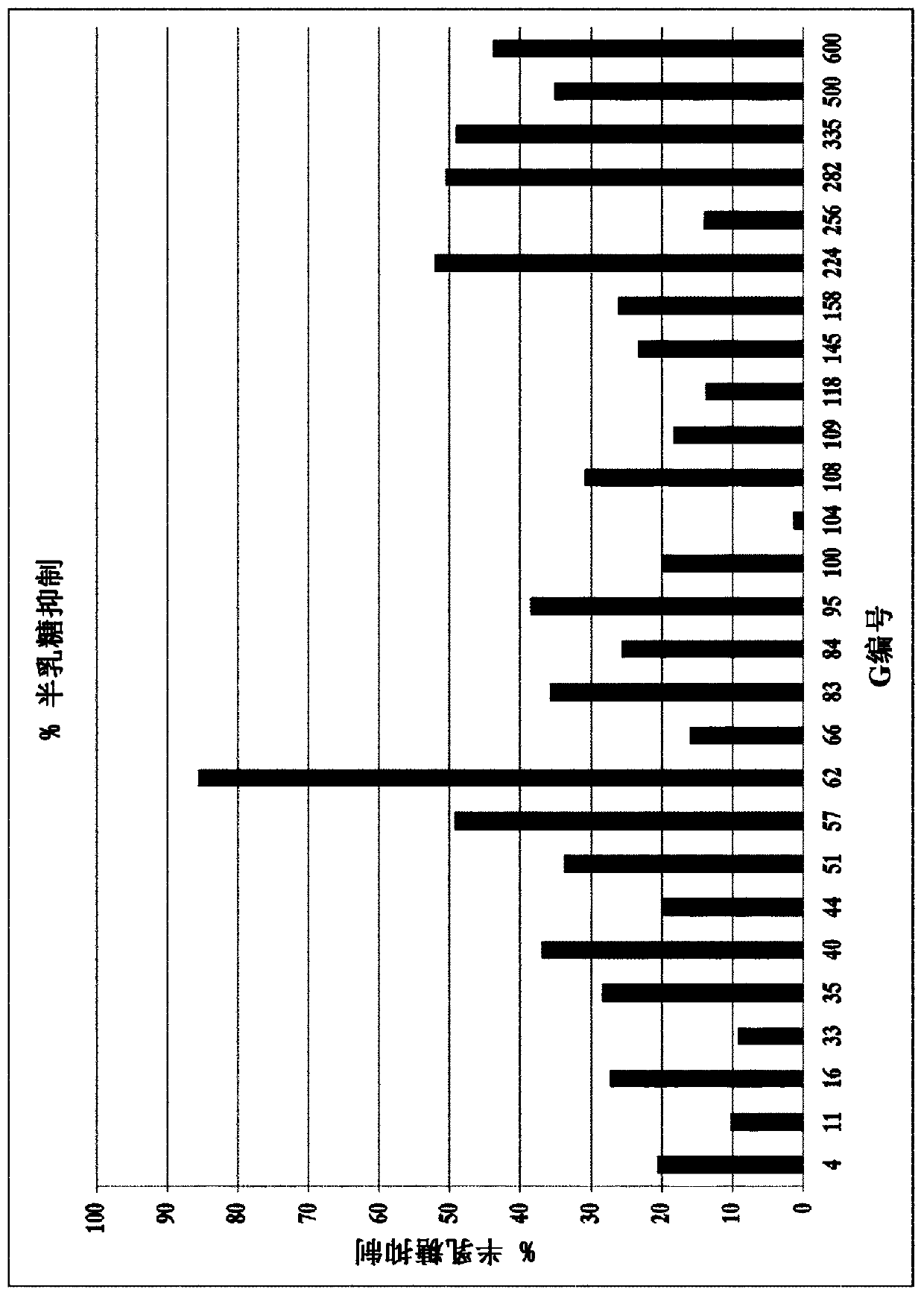
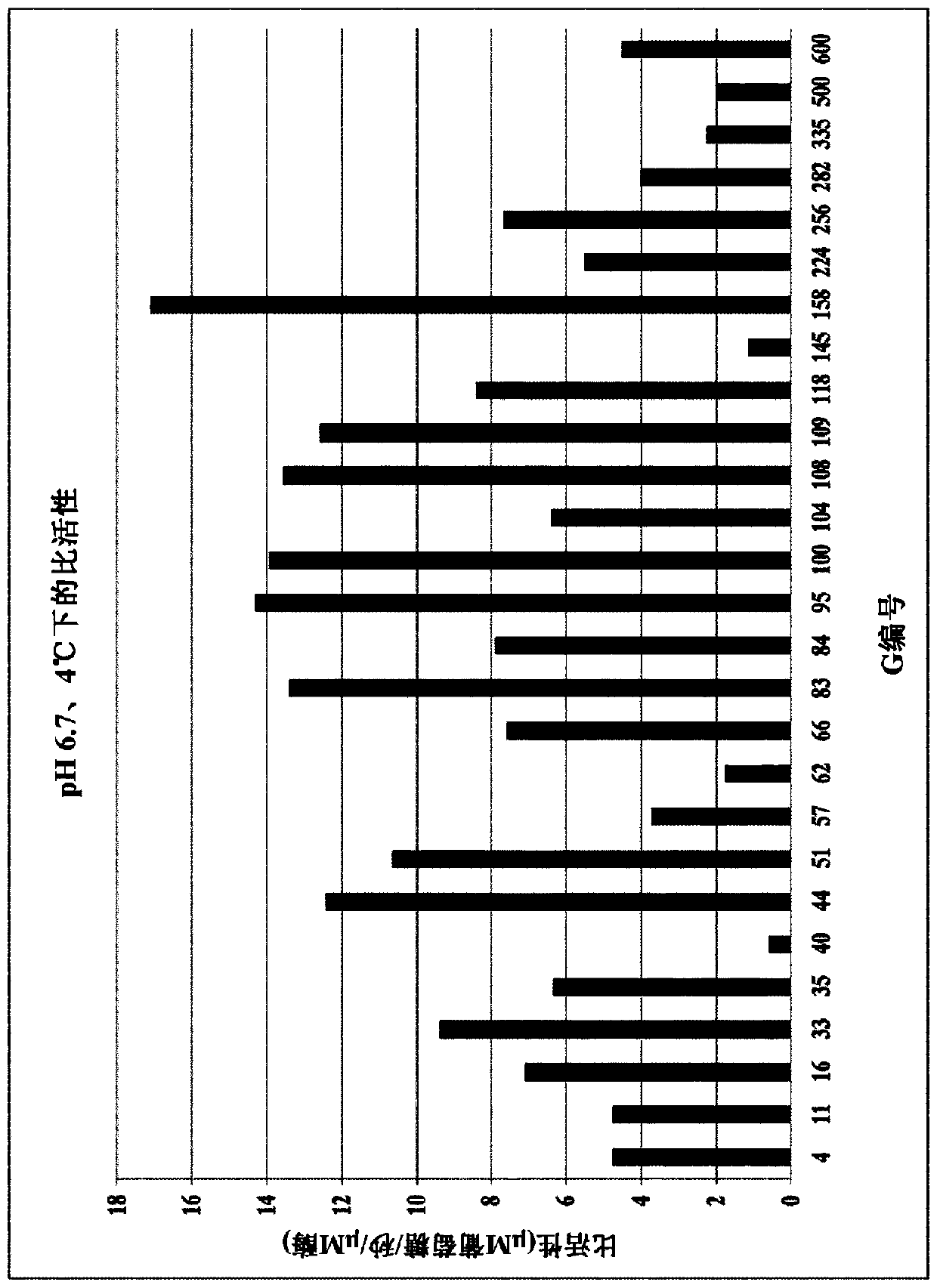
Lactase enzymes with improved properties
A technology of galactosidase and lactose, applied in the field of lactase with improved performance, can solve problems such as inappropriateness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0114] 1. A peptide exhibiting β-galactosidase activity, the peptide having SEQ ID NO: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, The amino acid sequence shown in 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33 or its enzymatically active fragment The sequence or any of the above sequences has no more than 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, Amino acid sequence with 21 or 22 amino acid substitutions, additions or deletions.
[0115] 2. A dimeric peptide exhibiting β-galactosidase activity, the composition of the dimeric peptide having SEQ ID NO: 2 and 3, 5 and 6, 20 and 21, 23 and 24, 26 and 27 or 28 Two peptides of the amino acid sequence shown in and 29; or enzymatically active fragments thereof, or any of the above sequences having no more than 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 , 13, 14, 15, 16, 17, 18, 19, 20, 21 or 22 amino acid substitution, addition or deletion amino acid sequence.
[0116] 3. The pepti...
Embodiment 1
[0241] Example 1: Construction of expression vector for producing lactase
[0242] Use a commercial genome extraction kit to extract genomic DNA of lactic acid bacteria or bifidobacteria according to the provided protocol (DNeasy, Qaigen, Germany). Using two synthetic primers, the purified genomic DNA source was used as the biomass to amplify the lactase gene by PCR. The PCR reagents were provided by the Phusion U Hot start DNA polymerase (Thermo Scientific, USA) kit. Use the USER cloning method (Nour-Eldin HH, Geu-Flores F, Halkier BA, PlantSecondary Metabolism Engineering, Methods in Molecular Biology, 643; 2010) to clone the lactase gene into the start codon of the expression vector pBAD / His to generate Expression construct. Using the USER cloning method, long complementary overhangs are generated on both the PCR product and the target vector. These overhangs can anneal to each other to form a stable hybrid product, which is used for transformation into E. coli without liga...
Embodiment 2
[0243] Example 2: Expression of lactase in E. coli expression host
[0244] The pBAD expression system was used to produce lactase in E. coli BW25113. A sterile loop was used to collect freshly transformed E. coli BW25113 cells carrying plasmid DNA from the Lb-Amp plate, and used to inoculate 5 mL of Lb-Amp medium. Use the overnight grown culture (200 μL) in a shaker ( 42) A 250 mL flask was inoculated with 50 mL of 2x PY medium (containing 100 μg / mL ampicillin). The culture was grown at 37°C and 220 rpm until the OD600 reached 0.6-0.8. The lactase expression was started by adding 0.05% arabinose to the medium, and the cells were cultured at 18° C. and 180 rpm for another 16-20 hours. The cells were collected by centrifugation (5000 rpm, 10 minutes at 4°C) and stored at -20°C until further use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com