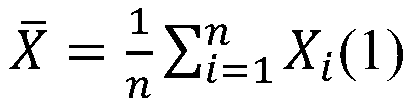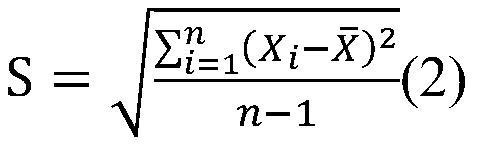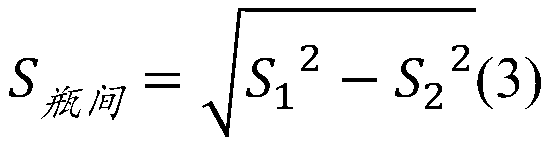Total complement activity quality control product and preparation method and application thereof, and reagent
A technology of complement and properties, applied in the field of biochemical and immune reagents, can solve the problem of lack of total complement activity, and achieve the effects of small differences between finished products, reduced errors, and simple methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] This embodiment provides a method for preparing a total complement activity quality control product, which specifically includes the following steps:
[0046]Choose pH 7.79, total protein content 43g / L, hemoglobin content 70g / L, sterility test negative, mycoplasma test negative, 56 ℃ inactivated 30 minutes newborn bovine serum. Add complement C1, complement C2, complement C3, complement C4, complement C5, complement C6, complement C7, complement C8 and complement C9 in the above-mentioned newborn bovine serum to make the first mixed solution, ensure the content of each complement component in the first mixed solution Concentrations are: Complement C130U / mL, Complement C215U / mL, Complement C3 15U / mL, Complement C4 15U / mL, Complement C5 15U / mL, Complement C6 15U / mL, Complement C7 15U / mL, Complement C8 15U / mL and Complement C9 15U / mL, then add Proclin300, 3% trehalose and 5% mannitol in the first mixed solution in the mass ratio of 0.02%; Complement Activity Quality Contr...
Embodiment 2
[0048] This embodiment provides a method for preparing a total complement activity quality control product, which specifically includes the following steps:
[0049] Choose pH 7.82, total protein content 45g / L, hemoglobin content 67g / L, sterility test negative, mycoplasma test negative, 56 ℃ inactivated 30 minutes newborn bovine serum. Add complement C1, complement C2, complement C3, complement C4, complement C5, complement C6, complement C7, complement C8 and complement C9 in the above-mentioned newborn bovine serum to make the first mixed solution, ensure the content of each complement component in the first mixed solution Concentrations are: Complement C160U / mL, Complement C230U / mL, Complement C3 30U / mL, Complement C4 30U / mL, Complement C5 30U / mL, Complement C6 30U / mL, Complement C7 30U / mL, Complement C8 30U / mL and Complement C9 30U / mL, and then add 0.02% sodium azide, 2% gelatin and 3% lactose in the first mixed solution in the first mixed solution; Complement Activity Qu...
Embodiment 3
[0051] This embodiment provides a method for preparing a total complement activity quality control product, which specifically includes the following steps:
[0052] Choose pH 7.66, total protein content 38g / L, hemoglobin content 58g / L, sterility test negative, mycoplasma test negative, 56 ℃ inactivated 30 minutes newborn bovine serum. Add complement C1, complement C2, complement C3, complement C4, complement C5, complement C6, complement C7, complement C8 and complement C9 in the above-mentioned newborn bovine serum to make the first mixed solution, ensure the content of each complement component in the first mixed solution Concentrations are: Complement C1100U / mL, Complement C250U / mL, Complement C3 50U / mL, Complement C4 50U / mL, Complement C5 50U / mL, Complement C6 50U / mL, Complement C7 50U / mL, Complement C8 50U / mL and Complement C9 50U / mL, and then adding thimerosal, 1.5% polyvinylpyrrolidone K30 and 3% sucrose in the first mixed solution in a mass ratio of 0.05%; low-tempera...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap