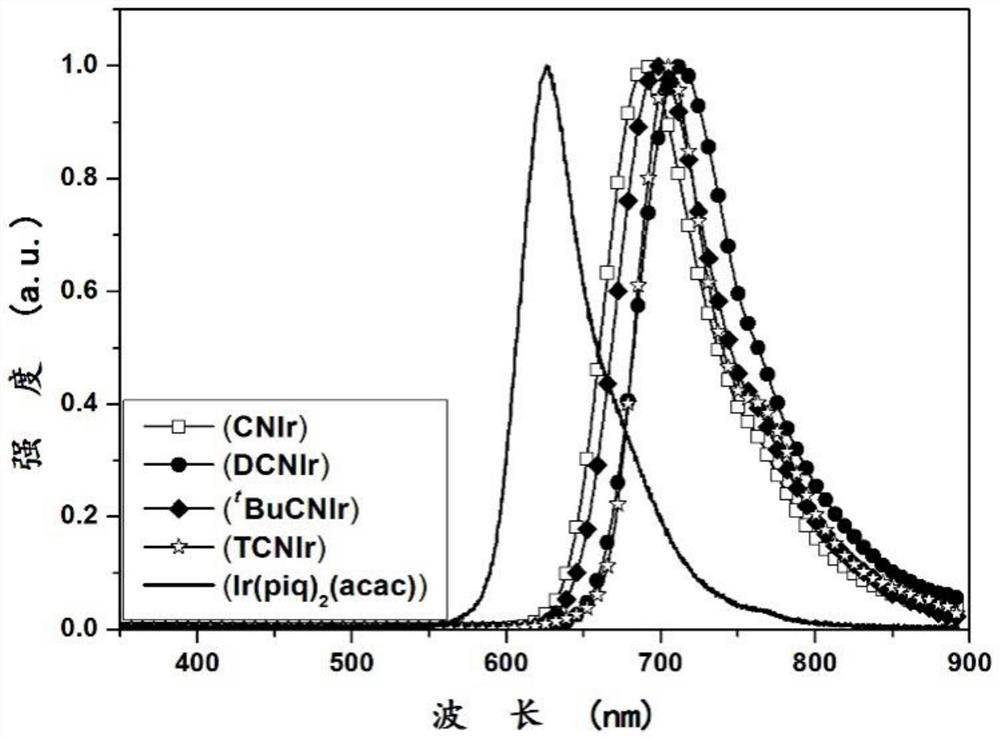
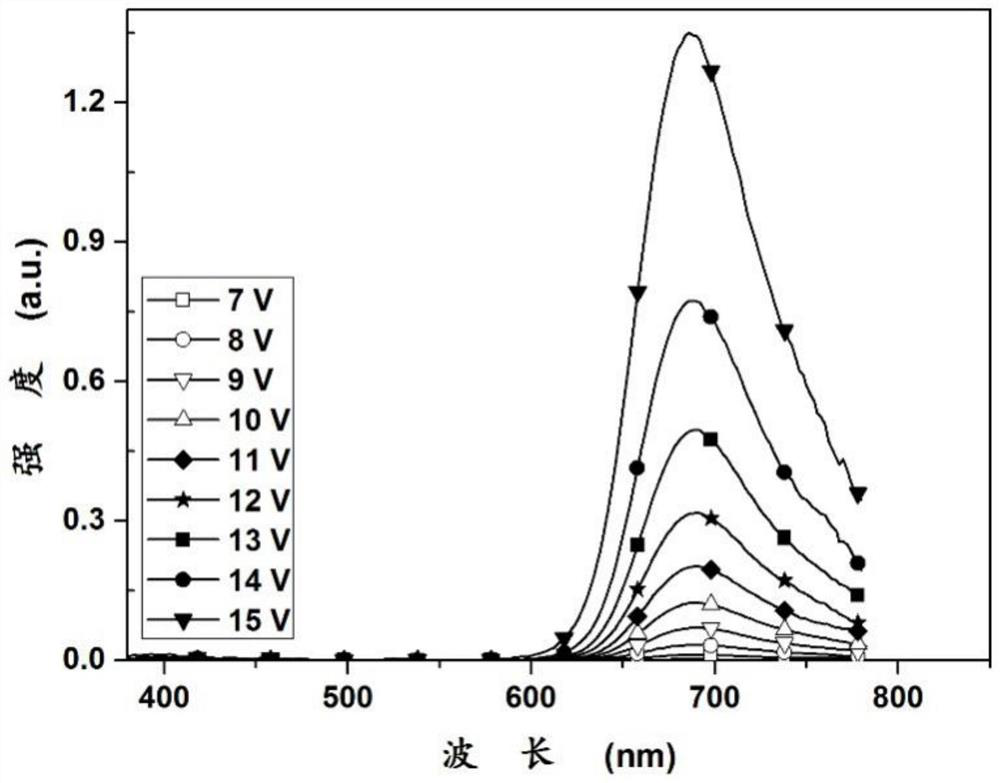
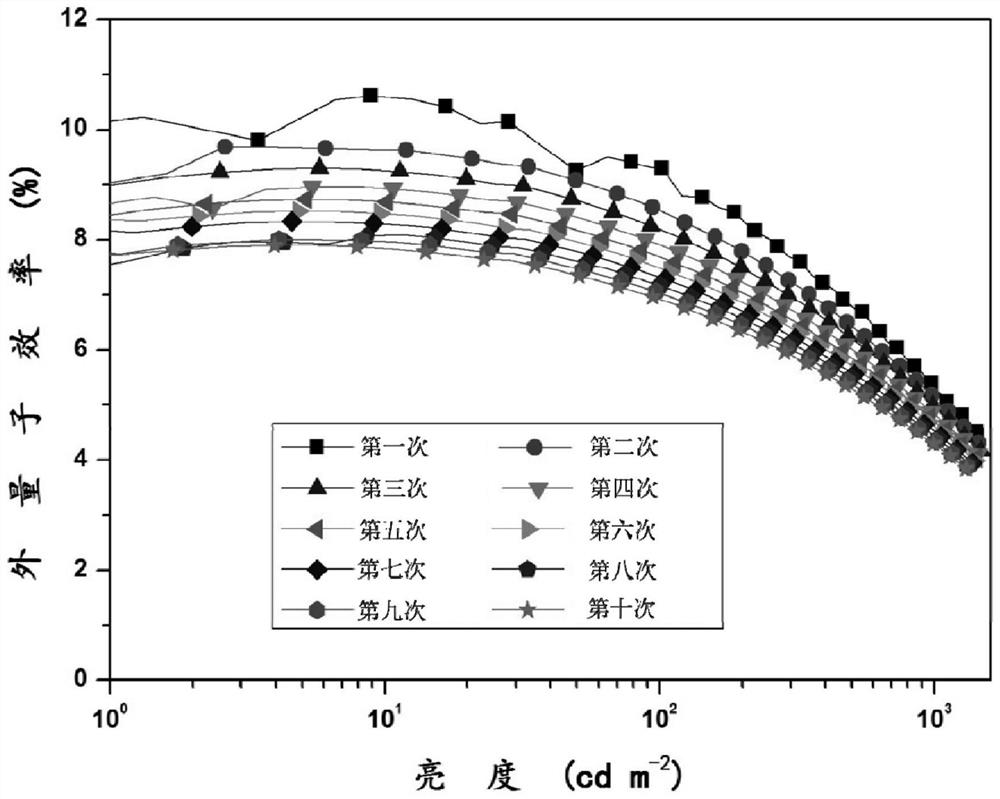
A kind of iridium complex as near-infrared luminescent material and its application
A technology of iridium complexes and luminescent materials, applied in luminescent materials, indium organic compounds, platinum group organic compounds, etc., can solve the problems of strengthening non-radiative decay of excited states, low luminous quantum efficiency of NIROLEDs, low external quantum efficiency, etc., to achieve Highlights Long half-life, broad commercialization prospects, and simple synthesis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the preparation of CNIr
[0031] (1) Preparation of 1-phenyl-4-cyanoisoquinoline:
[0032]
[0033] A 50ml flask was added with raw materials 1-chloro-4-cyanoisoquinoline (300mg, 1.59mmol), phenylboronic acid (290mg, 2.39mmol), potassium carbonate (439mg, 3.18mmol), palladium tetrakistriphenylphosphine (Pd(PPh 3 ) 4 ) (184mg, 0.16mmol), add 5ml of toluene, 0.75ml of ethanol and 2.5ml of water under the protection of nitrogen, and react at 95°C for 12 hours; then cool to room temperature, extract with ethyl acetate, pass through the column after rotary evaporation (ethyl acetate / Petroleum ether = 1 / 8, v / v) purification to obtain 241 mg of product, yield = 66%.
[0034] 1 H NMR (400MHz, CDCl 3 ,δ):8.98(s,1H),8.25(m,2H),7.92(m,1H),7.71(m,3H),7.57(m,3H);
[0035] 13 C NMR (100.5MHz, CDCl 3 ,δ): 165.0, 147.7, 138.3, 135.8, 132.7, 130.1, 129.9, 129.1, 128.8, 125.9, 124.7, 116.5, 104.9.
[0036] Matrix-assisted laser desorption ionization time-of-flig...
Embodiment 2
[0046] Embodiment 2: the preparation of t-BuCNIr:
[0047] (1) Preparation of 1-p-tert-butylphenyl-4-cyanoisoquinoline: similar to step (1) of Example 1, only the raw material phenylboronic acid was replaced with p-tert-butylphenylboronic acid. Yield = 76%.
[0048] 1 H NMR (400MHz, CDCl 3 ,δ):8.95(s,1H),8.30(d,J=8.4Hz,1H),8.25(d,J=8.4Hz,1H),7.9(m,1H),7.68(m,3H),7.59 (m,2H),1.40(s,9H);
[0049] 13 C NMR (100.5MHz, CDCl 3 ,δ): 164.9, 153.7, 147.6, 135.7, 135.4, 132.4, 129.9, 128.8, 125.8, 125.6, 124.5, 116.5, 104.5, 34.9, 31.3.
[0050] MALDI-TOF-MS: Molecular formula: Molecular formula: C 20 h 18 N 2 , Calculated: 286.3703; Measured: 287.0030.
[0051] (2) Preparation of dichloro(1-p-tert-butylphenyl-4-cyanoisoquinoline) iridium(III) dimer: similar to Example 1 step (2), only the raw material 1-chloro -4-cyanoisoquinoline was replaced by 1-p-tert-butylphenyl-4-cyanoisoquinoline.
[0052] (3) Preparation of t-BuCNIr: Similar to Example 1 step (3), only the raw mater...
Embodiment 3
[0056] Embodiment 3: the preparation of DCNIr:
[0057] (1) Preparation of 1-(3',5'-methylphenyl)-4-cyanoisoquinoline: Similar to step (1) in Example 1, only the raw material phenylboronic acid is replaced by 3,5-bis Tolylboronic acid. Yield = 65%.
[0058] 1 H NMR (400MHz, CDCl 3 ,δ):8.96(s,1H),8.25(dd,J=8.4Hz,0.8Hz,2H),7.92(m,1H),7.70(m,1H),7.30(s,1H),7.20(s ,1H),2.43(s,6H);
[0059] 13 C NMR (100.5MHz, CDCl 3 ,δ): 165.4, 147.5, 138.3, 138.1, 135.6, 132.5, 131.4, 128.9, 127.7, 125.9, 124.5, 116.4, 115.3, 104.6, 21.4.
[0060] MALDI-TOF-MS: Molecular formula: Molecular formula: C 18 h 14 N 2 , calculated value: 258.1157; measured value: 259.0741.
[0061] (2) Preparation of dichloro(1-(3',5'-methylphenyl)-4-cyanoisoquinoline) iridium(III) dimer: similar to step (2) of Example 1, Only the starting material 1-chloro-4-cyanoisoquinoline was replaced by 1-(3',5'-methylphenyl)-4-cyanoisoquinoline.
[0062] (3) Preparation of DCNIr: Similar to Example 1 step (3), only ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com