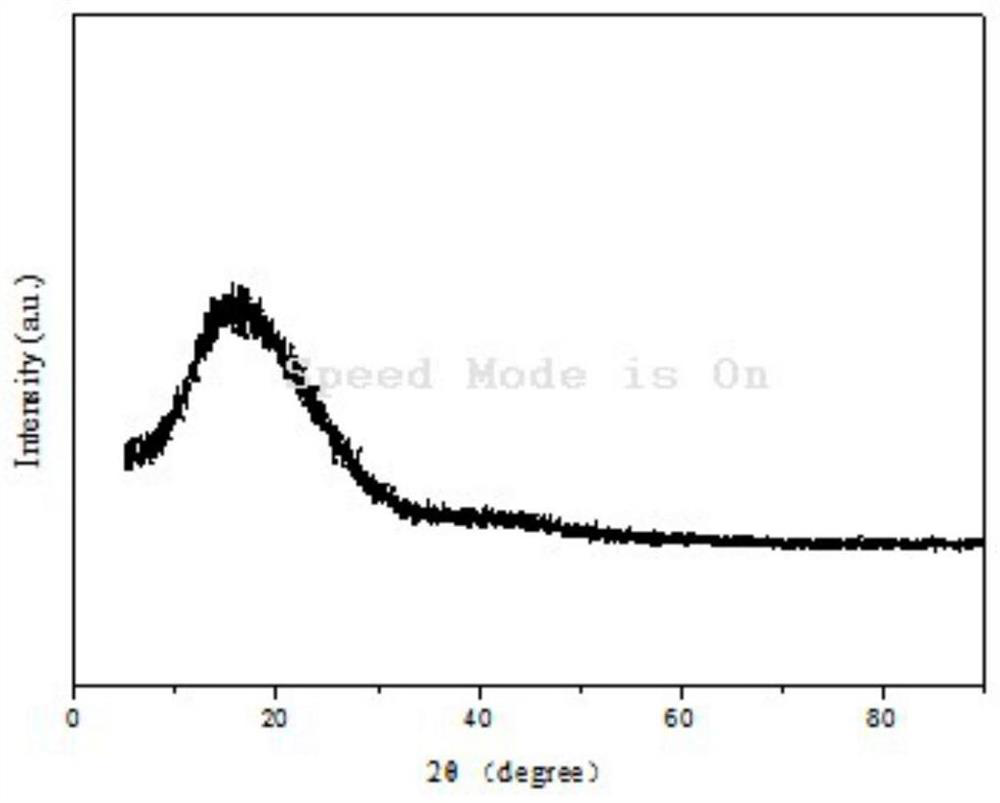
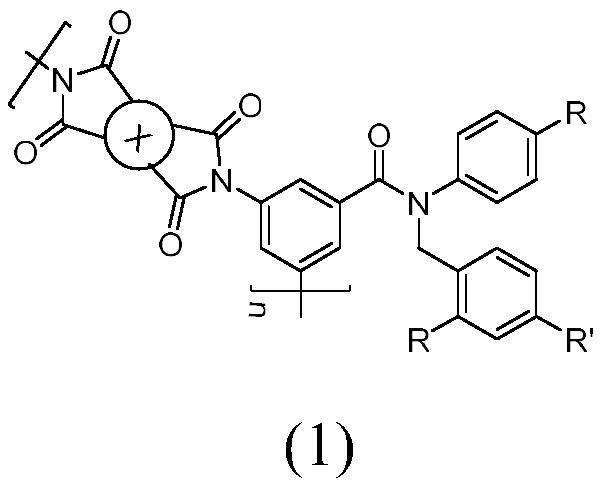
A kind of novel aromatic polyimide containing imide flexible group and preparation method thereof
A flexible imide and polyimide technology, applied in the field of new aromatic polyimide and its preparation, can solve the problem of polyimide synthesis steps, cumbersome post-processing, high cost and unfavorable large-scale industrial production and other problems, to achieve the effect of improving organic solubility, reducing conjugation effect, and large steric hindrance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1)N 2 Under protection, 3,5-diamino-N-(4'-bromophenyl)-N-(4"-bromobenzyl)benzamide (0.3g, 0.632mmol) was added to a 50ml three-necked flask, 3, 5-Dimethylphenylboronic acid (0.228g, 1.52mmol), Pd(PPh 3 ) 4 (0.0145g, 0.0126mmol), anhydrous potassium carbonate (0.349g, 2.53mmol), then add 15ml of toluene and 7.5ml of distilled water respectively, heat to 110°C, reflux and stir for about 5h, bubbles are constantly generated during the reaction, stir for 5h Finally, close the reaction, let it cool down to room temperature naturally, filter under reduced pressure, separate the obtained filtrate, collect the toluene layer, and remove the solvent toluene by rotary evaporation to obtain a white solid, which is combined with the filter cake and purified by column chromatography. , the solvents used are ethyl acetate and n-hexane, and the polarity ratio is ethyl acetate:n-hexane=5:1, and the resulting product is dried in vacuum at 90°C for 12h to obtain 3,5-diamino-N-(4'-3 ",...
Embodiment 2
[0046] (1)N 2 Under protection, 3,5-diamino-N-(4'-bromophenyl)-N-(4"-bromobenzyl)benzamide (0.3g, 0.632mmol) was added to a 50ml three-necked flask, 3, 5-Dimethylphenylboronic acid (0.228g, 1.52mmol), Pd(PPh 3 ) 4 (0.0145g, 0.0126mmol), anhydrous potassium carbonate (0.349g, 2.53mmol), then add 15ml of toluene and 7.5ml of distilled water respectively, heat to 110°C, reflux and stir for about 5h, bubbles are constantly generated during the reaction, stir for 5h Finally, close the reaction, let it cool down to room temperature naturally, filter under reduced pressure, separate the obtained filtrate, collect the toluene layer, and remove the solvent toluene by rotary evaporation to obtain a white solid, which is combined with the filter cake and purified by column chromatography. , the solvents used are ethyl acetate and n-hexane, and the polarity ratio is ethyl acetate:n-hexane=5:1, and the resulting product is dried in vacuum at 90°C for 12h to obtain 3,5-diamino-N-(4'-3 ",...
Embodiment 3
[0053] (1) Under nitrogen protection, add 3,5-diamino-N-(4′-bromophenyl)-N-(4”-bromobenzyl)benzamide (0.3g, 0.632 mmol), 4-(diphenylamino)phenylboronic acid (0.44g, 1.52mmol), Pd(PPh 3 ) 4 (0.0145g, 0.0126mmol), anhydrous potassium carbonate (0.349g, 2.53mmol), then add 15ml of toluene and 7.5ml of distilled water respectively, heat to 110°C, reflux and stir for about 5h, bubbles are constantly generated during the reaction, stir for 5h Finally, close the reaction, let it cool down to room temperature naturally, filter under reduced pressure, separate the obtained filtrate, collect the toluene layer, and remove the solvent toluene by rotary evaporation to obtain a white solid, which is combined with the filter cake and purified by column chromatography. , the solvents used are ethyl acetate and n-hexane, and the polarity ratio is ethyl acetate:n-hexane=5:1, and the resulting product is dried in vacuum at 90°C for 12h to obtain 3,5-diamino-N-(4'-benzene Base-4"-triphenylamino...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| surface roughness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com