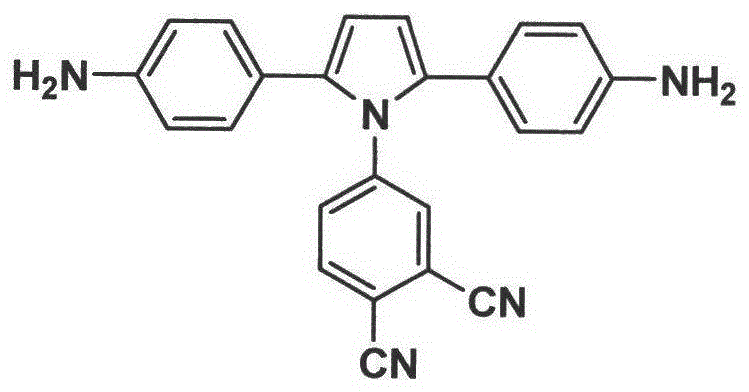
Pyrrolyl aromatic diamine containing phthalonitrile structure and its preparation method and application
A technology of pyrrolyl aromatic diamine and phthalonitrile, which is applied in the field of polymer preparation to achieve the effects of improving flame retardancy and organic solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
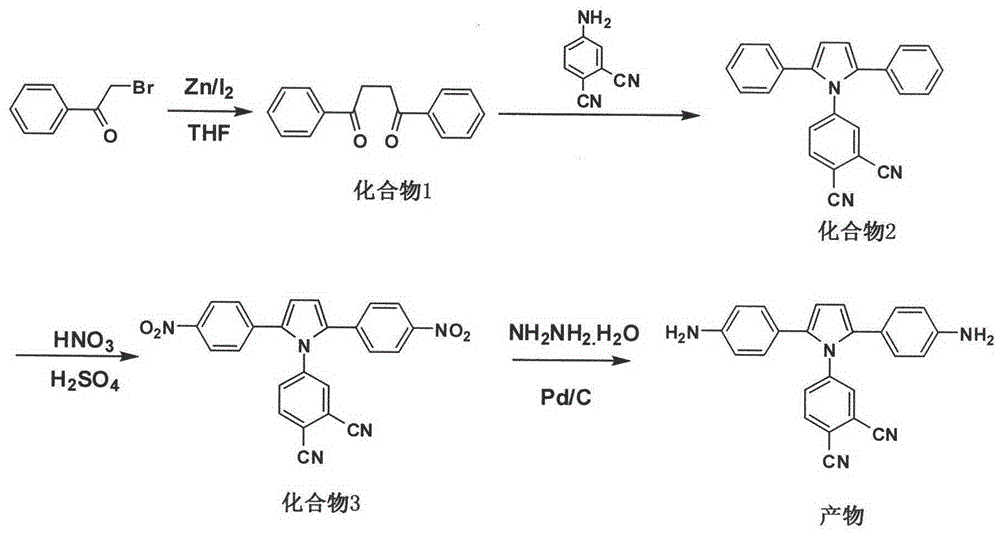
[0050] Example 1: Preparation of 1,4-diphenyl-1,4-diketone (compound 1)
[0051] 30.0g anhydrous tetrahydrofuran and 3.0g (15mmol) 2-bromoacetophenone were successively added to the reaction kettle with stirrer, thermometer and nitrogen replacement device, and 1.0g (15mmol) zinc powder and 0.1 g iodine. React at 66°C for 17 hours; after the reaction, filter the insoluble matter, slowly pour the filtrate into water, extract the organic phase with chloroform and wash with water, extract the chloroform by rotary evaporation and dry it with anhydrous magnesium sulfate, and use chloroform / n-hexane with a volume ratio of 8:2 The white needle-like solid is 1,4-diphenyl-1,4-dione after separation by eluent column chromatography, the yield is about 65-80%, and the melting point is 143-145°C. Infrared spectrum FT-IR (KBr tablet): 3081, 3071, 1677, 1577, 989, 761. H NMR spectrum test 1 H NMR (CDCl 3 , 200Hz) δ: 3.46 (s, 4H), 7.50 (m, 6H), 8.04 (m, 4H).
Embodiment 2
[0052] Example 2: Preparation of 4-(2,5-diphenyl-1H-pyrrolyl)phthalonitrile (compound 2)
[0053] 800mL dry toluene, 23.8g (100mmol) 1,4-diphenyl-1,4-dione (compound 1), 17.2g (120mmol) 4-aminophthalonitrile and 2.1g (12mmol) Add p-toluenesulfonic acid into a reaction kettle equipped with a stirrer, thermometer and nitrogen replacement device, raise the temperature to reflux under normal pressure; react at reflux temperature for 120h; after the reaction, evaporate toluene, wash with water and dry over anhydrous magnesium sulfate , using dichloroethane / n-hexane with a volume ratio of 1:1 as eluent column chromatography to obtain a gray solid that is 4-(2,5-diphenyl-1H-pyrrolyl)phthalonitrile, producing The rate is about 78-86%; H NMR spectrum test 1 H NMR (400MHz, CD 3 Cl) δ: 6.53 (s, 2H), 7.40 (m, 2H), 7.56 (m, 4H), 7.72 (m, 5H), 7.81 (d, 1H), 8.09 (d, 1H). C 24 h 15 N 3 (345) Elemental analysis theoretical value: C, 83.46; H, 4.38; N, 12.17; measured value: C, 83.51; H,...
Embodiment 3
[0054] Example 3: Preparation of 4-(2,5-bis(4-nitrophenyl)-1H-pyrrolyl)phthalonitrile (compound 3)
[0055] Mix 10mL of concentrated nitric acid with a mass concentration of 70% and 12mL of concentrated sulfuric acid with a mass concentration of 98% to form a mixed acid solution, add it to a reaction kettle equipped with a stirrer, a thermometer and a nitrogen replacement device, and cool it to 0°C; then add dropwise 22 mL of a nitromethane solution of 2M 4-(2,5-diphenyl-1H-pyrrolyl)phthalonitrile to this mixed acid solution, and keep the system temperature at 0°C; after the dropwise addition, react at 0°C for 2h and 35°C for 2h; after the reaction, slowly pour the reaction solution into crushed ice to obtain a light yellow solid, which is washed with water until neutral and collected by filtration; 1:1 methanol / toluene mixed solvent recrystallization, vacuum drying to obtain 4-(2,5-bis(4-nitrophenyl)-1H-pyrrolyl)phthalonitrile with a yield of about 72-74 %; H NMR spectrum te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com