Phthalonitrile derivative, preparation method thereof, metal phthalocyanine derivative and preparation method and application thereof
A technology of phthalocyanine and metal phthalocyanine, applied in the field of hole transport materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The present invention also provides a method for preparing the phthalocyanine derivative described in the above technical scheme, comprising the following steps: in a protective atmosphere, 4-bromo-phthalonitrile and 4-boronic acid ester-4', 4'-dimethoxytriphenylamine undergoes a coupling reaction to obtain 4-phthalocyano-4',4'-dimethoxytriphenylamine. The reaction formula is shown in formula (1):
[0033]
[0034] In the present invention, the mass ratio of 4-bromo-phthalonitrile to 4-boronate-4',4'-dimethoxytriphenylamine is preferably 1:4-5.
[0035] In the present invention, the solvent of the coupling reaction is preferably a mixed solution of water and tetrahydrofuran, and the volume ratio of the water and tetrahydrofuran is preferably 3:50; the catalyst used in the coupling reaction is preferably tetrakis(triphenylphosphine ) palladium, the mass ratio of the catalyst to 4-bromo-phthalonitrile is preferably 1:0.5~0.7; the coupling reaction is preferably carrie...
Embodiment 1
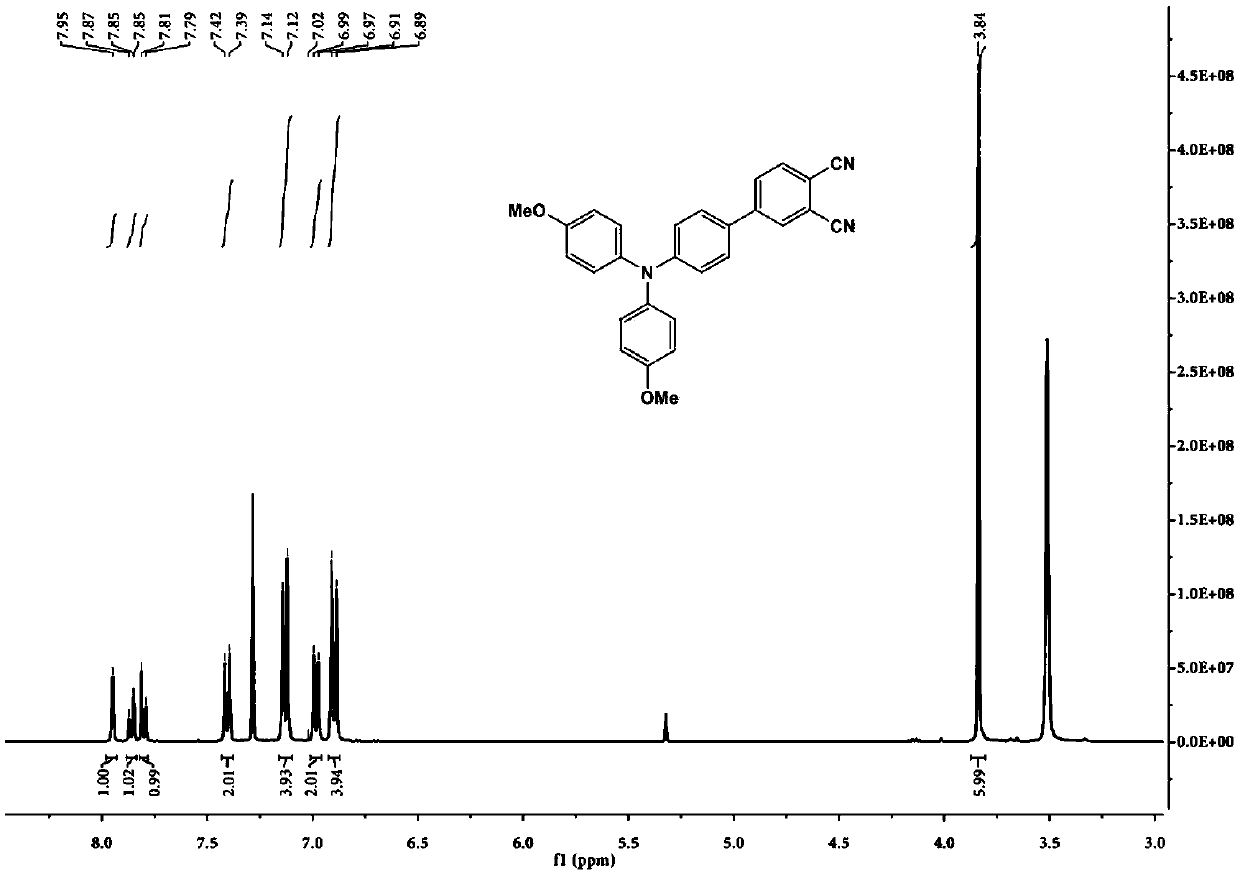
[0070] 4-Bromo-phthalonitrile (0.414g), 4-boronate-4',4'-dimethoxytriphenylamine (1.725g), tetrakis(triphenylphosphine)palladium (0.231g) and Potassium carbonate (2.764g) was placed in a mixed solution of water (3mL) and tetrahydrofuran (50mL), and nitrogen was used as a protective gas, and inflated-degassed three times to obtain a nitrogen protective atmosphere, and then kept stirring at 80°C for 12h, and the reaction was completed Afterwards, the reaction solution was cooled to room temperature, and magnesium sulfate was added to remove water; the solution after water removal was spin-dried to obtain a solid product, and an eluent (petroleum ether: ethyl acetate: the volume ratio of dichloromethane was 20:1: 2) The obtained solid product is subjected to column chromatography; after the column chromatography is completed, the obtained solution is spin-dried to obtain an o-phthalocyanine derivative. According to nuclear magnetic analysis, its structure is shown in formula I, wh...
Embodiment 2
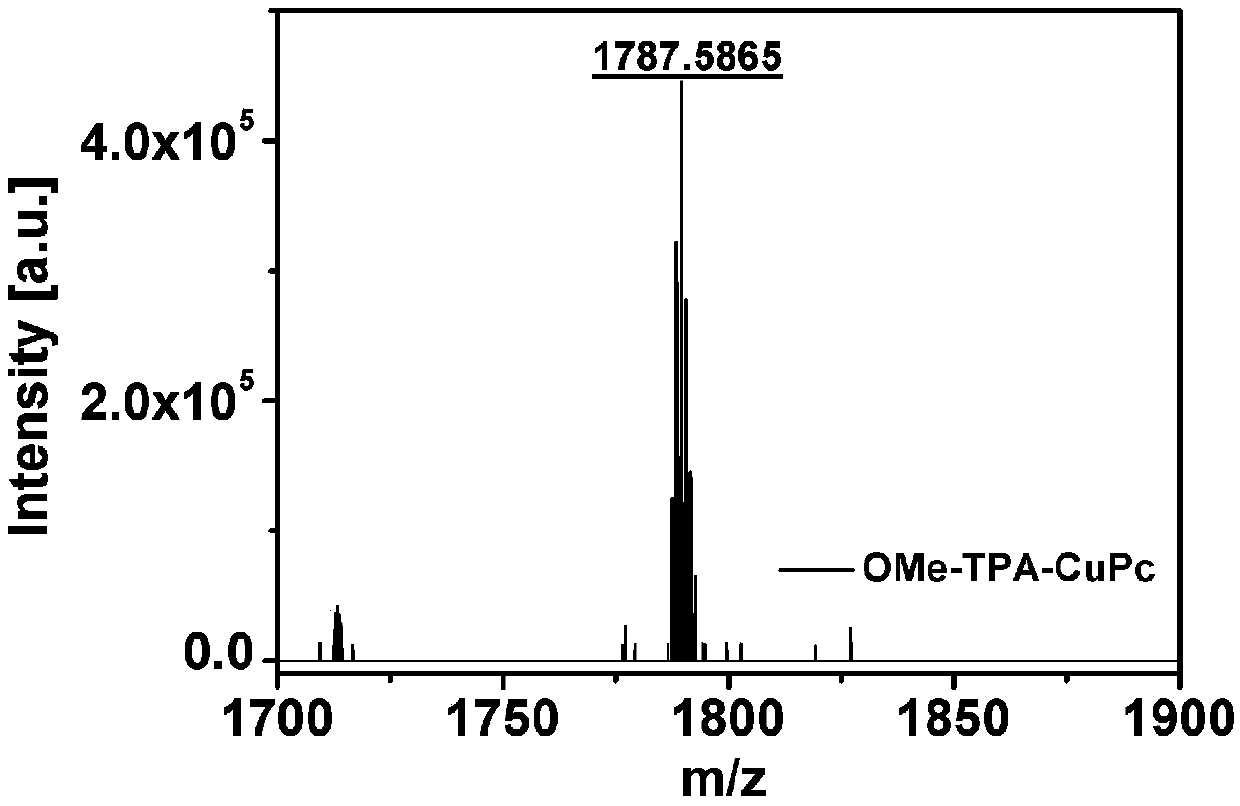
[0073]The phthalocyanine derivative (1g) obtained in Example 1 and anhydrous cuprous chloride (104mg) were mixed according to a molar ratio of 3:1 and placed in 5mL of n-hexanol, and 0.5mL of 1,8-diazabicyclo [5.4.0] Undec-7-ene, using nitrogen as a protective gas, inflated-degassed three times to obtain a protective gas atmosphere, and then kept stirring at 150 ° C for 12 hours. After the reaction was completed, the reaction solution was cooled to room temperature. The cooled reaction solution was subjected to column chromatography, and eluted using an eluent (the volume ratio of petroleum ether, ethyl acetate and methanol was 10:1:0.5); the solution obtained by column chromatography was evaporating to dryness, and the product Transfer to the Soxhlet extractor, use ethanol as the solvent, wash at 120°C, after 20 hours, the color of the solvent turns yellow and dark, replace the solvent and repeat the operation 3 to 5 times; place the product of the previous step in a mixed sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com