Modified oligomeric compounds comprising tricyclo-DNA nucleosides and uses thereof
A technology of oligomeric compounds and nucleosides, applied in the field of modified oligomeric compounds containing tricyclic DNA nucleosides and their uses, can solve the problems of acute toxicity, preferably long-term toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0741] Compositions of the invention for treating Duchenne muscular dystrophy
[0742] Effectiveness evaluation
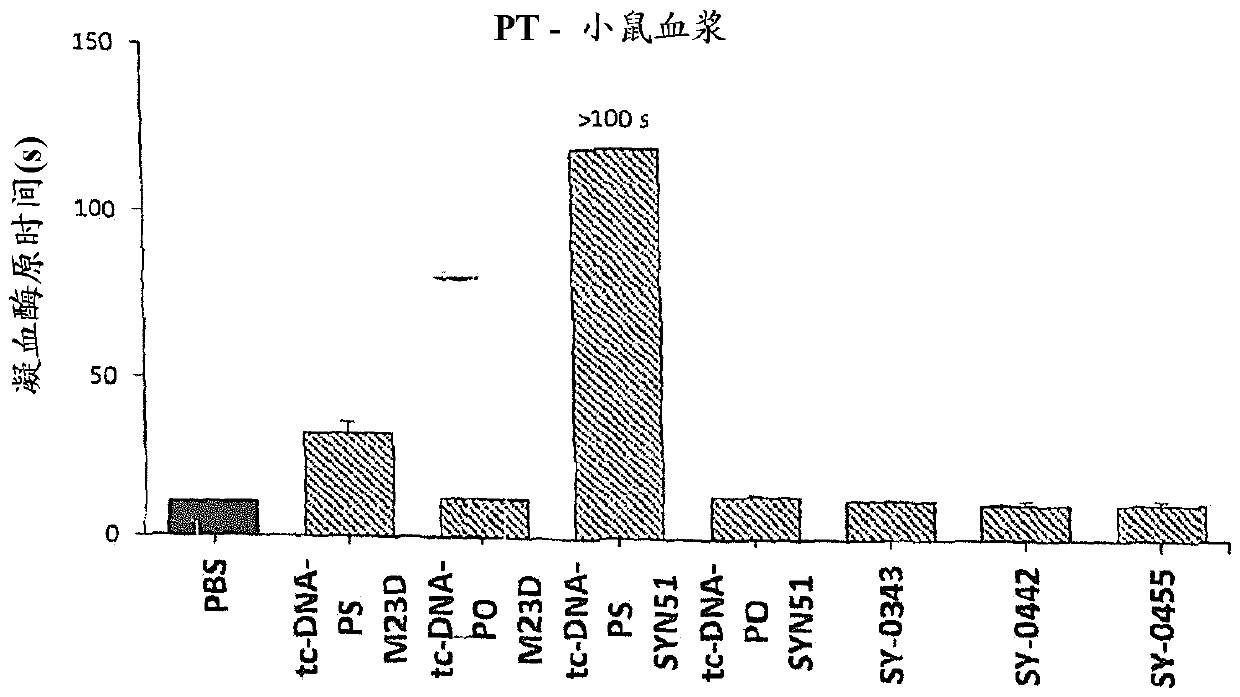
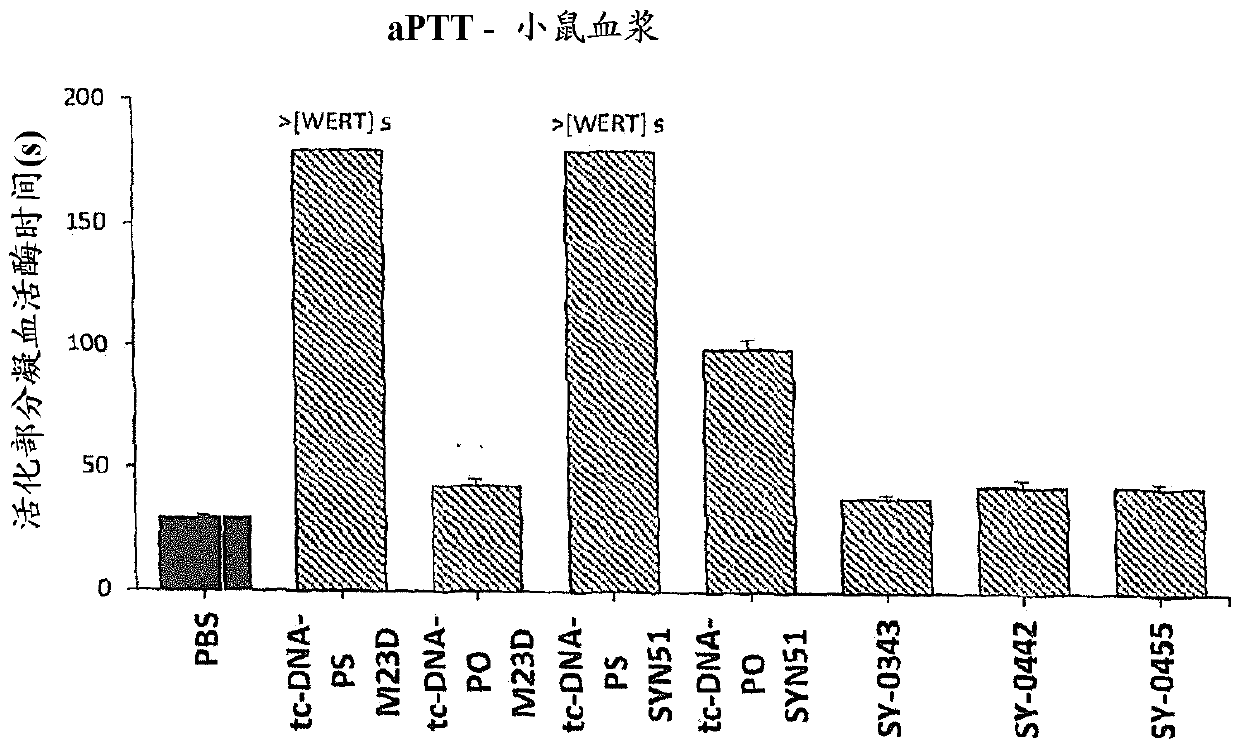
[0743] Adult mdx mice were injected weekly for 4 weeks with different 13-mer AONs targeting the donor splice site (M23D: +2-11) of exon 23 of dystrophin pre-mRNA (i.e. SY-0308, SY-0210 and SY-0299, SY-0343, SY-0442 and SY-0455 of the present invention) treatment. SY-0308 (also referred to herein interchangeably as "tcDNA-PO M23D") corresponds to p-CCTCGGCTTACCT-OH of SEQ ID NO: 1, wherein all nucleotides are tc-DNA, and all internucleoside linkages The group is a phosphodiester linking group, and p is the phosphate moiety at the 5' end. SY-0210 (also referred to herein interchangeably as "tcDNA-PS M23D") corresponds to p-CCTCGGCTTACCT-OH of SEQ ID NO: 1, wherein all nucleotides are tc-DNA, and all internucleoside linkages The group is a phosphorothioate linking group, and p is the phosphate moiety at the 5' end. Composition SY-0343 of the present invention, int...
Embodiment 2
[0765] Co-precipitation experiments and proteomics
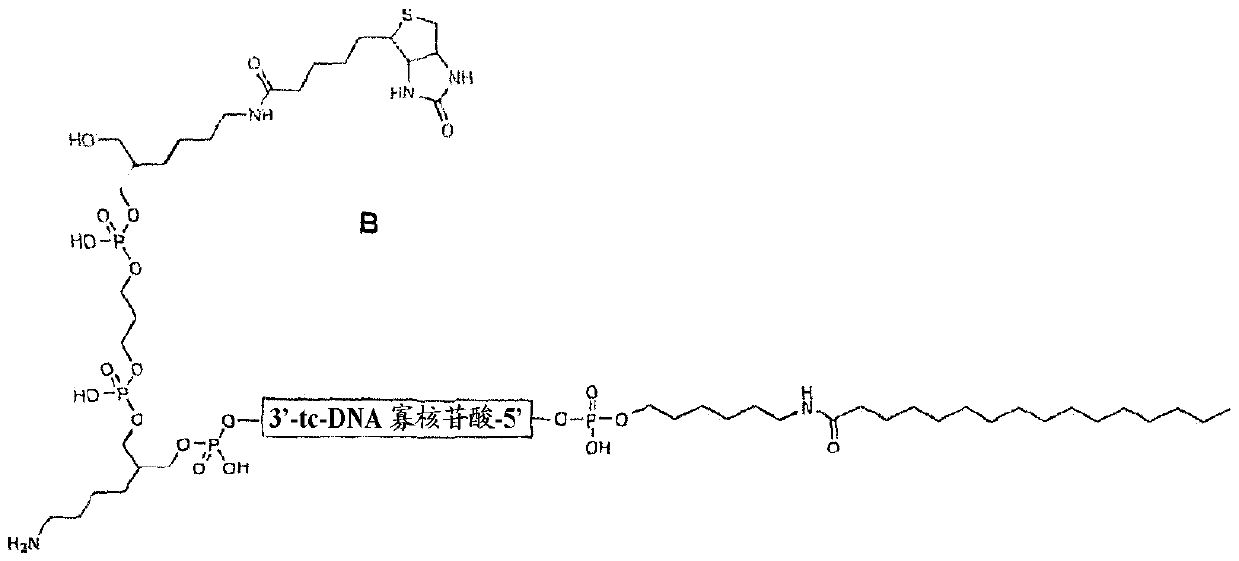
[0766] Target oligonucleotides were synthesized as biotinylated conjugates for subsequent co-precipitation of potentially interacting serum proteins by using streptavidin beads. The biotinylated derivatives used in this study were SY-0440, SY-0427, SY-0446, SY-0448, SY-0445, SY-0443 and SY-0451, which are defined and characterized in Table 3 and as Figure 6 shows.
[0767] Biotinylated oligonucleotides immobilized on streptavidin beads were incubated in the presence of serum for 1 hour. Beads were collected by low speed centrifugation, washed and then dissolved in appropriate buffer for further protein analysis by SDS-PAGE, Orbitrap LC-MS / MS and SDS-PAGE / MALDI-TOF.
[0768] Figure 7SDS-PAGE analysis of proteins recovered from mouse and human sera is illustrated for the oligonucleotides used in this study. It appears that SY-0440 (i.e., tcDNA M23D with full PS) retains much more serum protein than its tcDNA-PO equivalent...
Embodiment 3
[0778] Stability of the composition of the present invention in human serum
[0779] The stability of preferred compositions of the invention in human serum was studied based on oligonucleotides SY-0343, SY-0442, SY-0299, SY-0450, SY-0455 and SY-0357. Table 3 defines and characterizes the compositions of the invention.
[0780] Prepare a mixture (total volume 80 µL) consisting of 40 µL of human serum, oligonucleotide stock solution, 20 µL of PBS (2x), and milliQ water in a 0.2 mL PCR vial such that the final concentration of oligonucleotide is 2 µM , and incubated at 37°C. Aliquots were taken after 4, 24 and 120 hours. Digest the mixture with proteinase K in a PCR vial for 2 h at 55 °C (80 µL of the mixture, 100 µL of proteinase K buffer 2x (200 mM Tris-HCl (pH 8.5), 400 mM NaCl, 10 mM EDTA, 0.4% SDS ), 20 µL proteinase K (20 mg / mL in 100 mM Tris-HCl (pH 8.5), 10 mM CaCl 2 middle). The mixture was then cooled to room temperature, centrifuged (13400 rpm, 10 minutes) and th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap