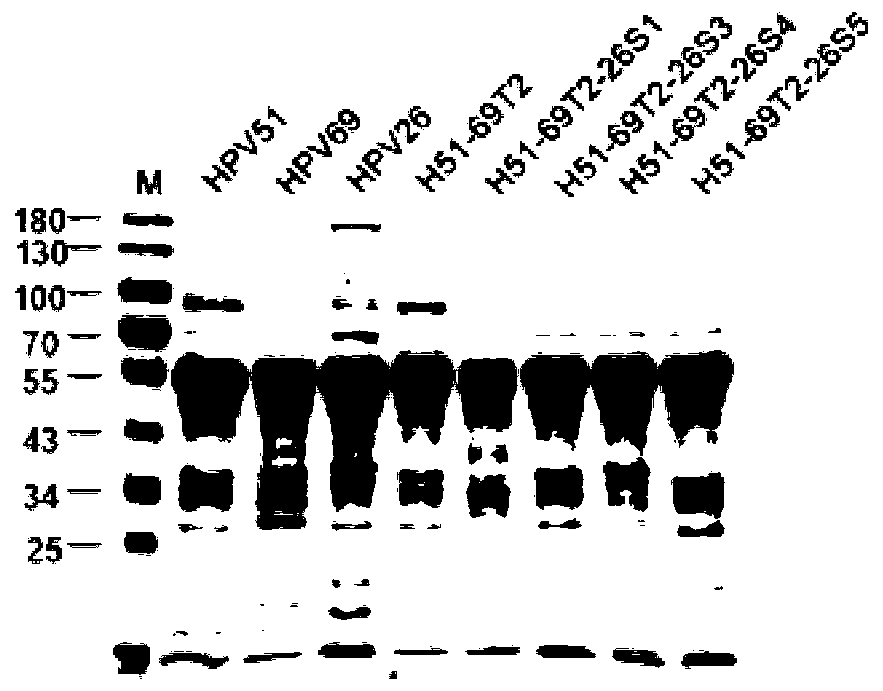
Mutant of human papillomavirus type 51 L1 protein
A technology of L1 protein and protein, applied in the field of molecular virology and immunology, can solve the problems of safety and HPV vaccine production cost increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0262] Example 1. Expression and purification of mutated HPV51 L1 protein
[0263] Construction of expression vector
[0264] Using Gibson assembly (Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods. 2009; 6:343–5. doi:10.1038 / nmeth.1318 .) to construct an expression vector encoding a mutated HPV51 L1 protein, which contains a specific segment derived from HPV69 L1 and / or a specific segment derived from HPV26L1. In short, PCR reaction is first used to obtain a short fragment containing a mutation and a long fragment not containing a mutation, and then the Gibson assembly system is used to link the two fragments into a circle.
[0265] The initial templates used include pTO-T7-HPV51N9C plasmid (the HPV51L1 protein with 9 amino acid truncated at its coding N-terminal; abbreviated as 51L1N9 in Table 2), pTO-T7-HPV69N0C plasmid (its coding full-length HPV69 L1 protein; Abbreviated ...
Embodiment 2
[0309] Example 2: Assembly of HPV virus-like particles and detection of particle morphology
[0310] Assembly of HPV virus-like particles
[0311] Take a certain volume (about 2ml) of protein H51N9-69T1, H51N9-69T2, H51N9-69T3, H51N9-69T4, H51N9-69T5, H51N9-69T1-26S2, H51N9-69T1-26S3, H51N9-69T1-26S4, H51N9-69T1 -26S5, H51N9-69T2-26S1, H51N9-69T2-26S3, H51N9-69T2-26S4, H51N9-69T2-26S5, H51N9-69T3-26S1, H51N9-69T3-26S2, H51N9-69T3-26S4, H51N9-69T , H51N9-69T4-26S1, H51N9-69T4-26S2, H51N9-69T4-26S3 and H51N9-69T4-26S5, respectively dialyzed to (1) 2L storage buffer (20mM sodium phosphate buffer pH 6.5, 0.5M NaCl); (2) 2L refolding buffer (50mM sodium phosphate buffer pH 6.0, 2mM CaCl 2 , 2mM MgCl 2 , 0.5M NaCl); and (3) 20 mM sodium phosphate buffer pH 7.0, 0.5M NaCl. Dialysis was performed for 12 h in each of the three buffers.
[0312] By similar method, HPV51N9, HPV69N0 and HPV26N0 proteins were assembled into HPV51N9 VLP, HPV69N0 VLP and HPV26N0 VLP, respectively.
...
Embodiment 3
[0319] Example 3: Evaluation of neutralizing antibody titers in mouse serum after immunization with virus-like particles 1
[0320] In this experiment, the virus-like particles used were: H51N9-69T1 VLP, H51N9-69T2 VLP, H51N9-69T3 VLP, H51N9-69T4 VLP and H51N9-69T5 VLP.
[0321] In this experiment, the immunization scheme is shown in Table 4. All mice (6-week-old BalB / c female mice) were divided into 2 groups: aluminum adjuvant group 1 (immunization dose was 5 μg, using aluminum adjuvant), aluminum adjuvant group 2 (immunization dose was 1 μg, using aluminum adjuvant). Each group was further subdivided into 8 subgroups, control subgroups 1 and 2 were immunized with HPV51N9 VLP alone and HPV69N0 VLP alone, and control subgroup 3 was immunized with mixed HPV51 / HPV69 VLP (i.e., HPV51N9 VLP and HPV69N0 VLP mixture, wherein each VLP is administered at the specified immunization dose), experimental subgroups 1, 2, 3, 4, and 5 were immunized with H51N9-69T1 VLP, H51N9-69T2 VLP, H51...
PUM
| Property | Measurement | Unit |
|---|---|---|
| sedimentation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com