Method for reducing NAD analogues by utilizing methanol
An analogue, methanol technology, applied in the direction of fermentation, etc., can solve the problem of transforming methanol dehydrogenase that has not been reported in the literature, and achieve the effect of selective delivery, abundant reserves, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Using methanol as a reducing agent, methanol dehydrogenase catalyzes the reduction of NAD analogs
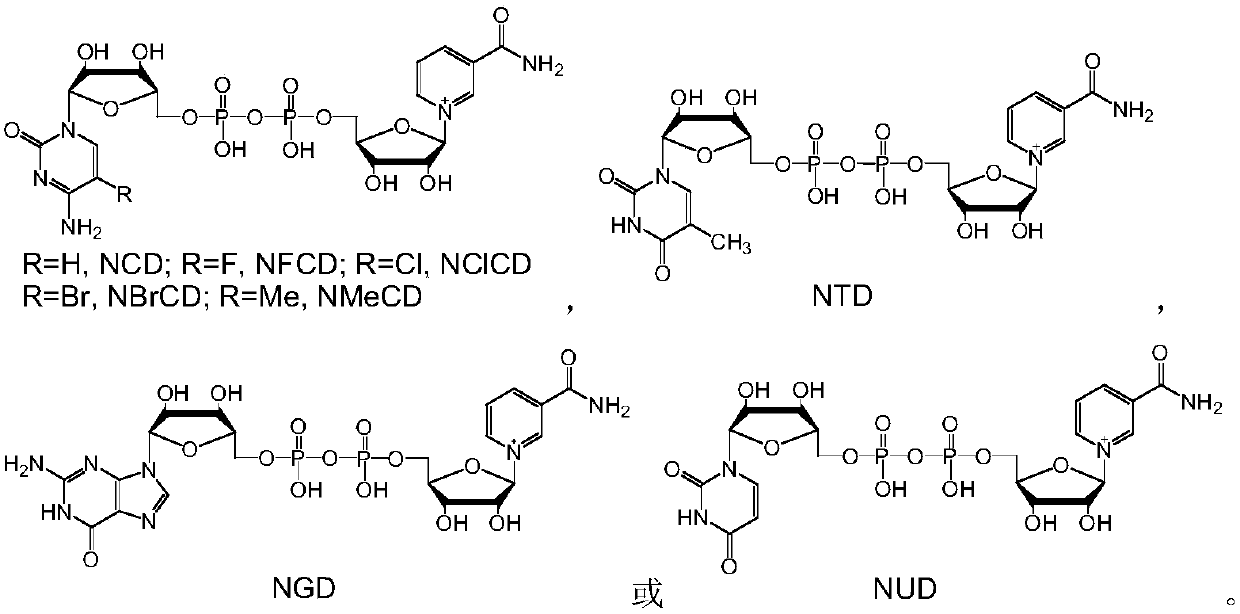
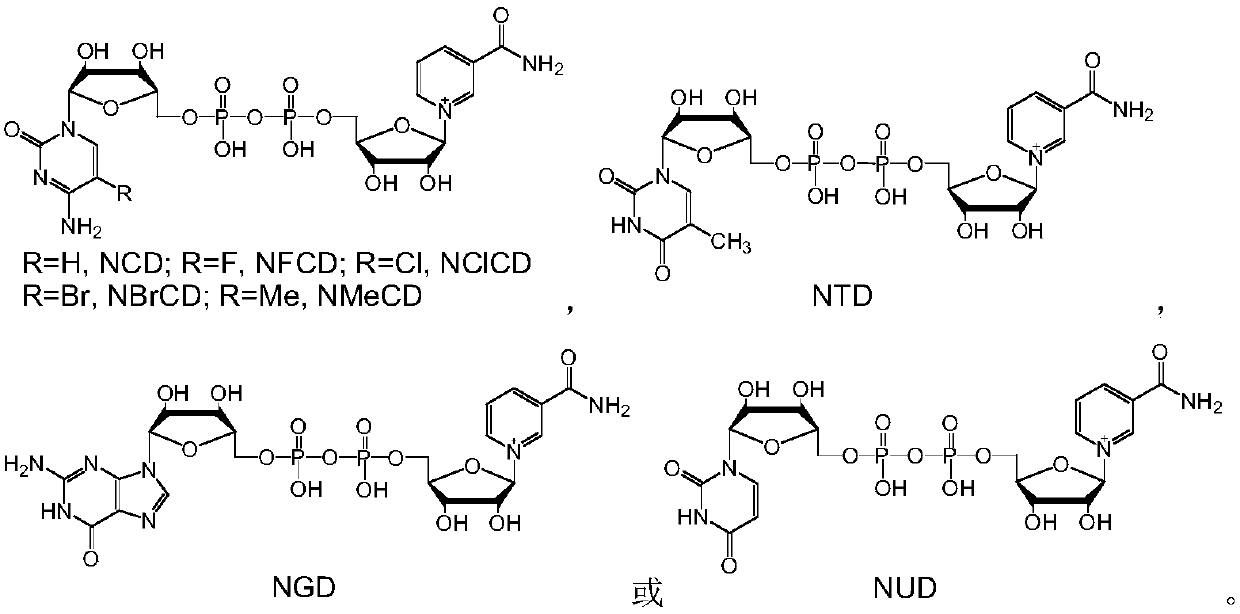
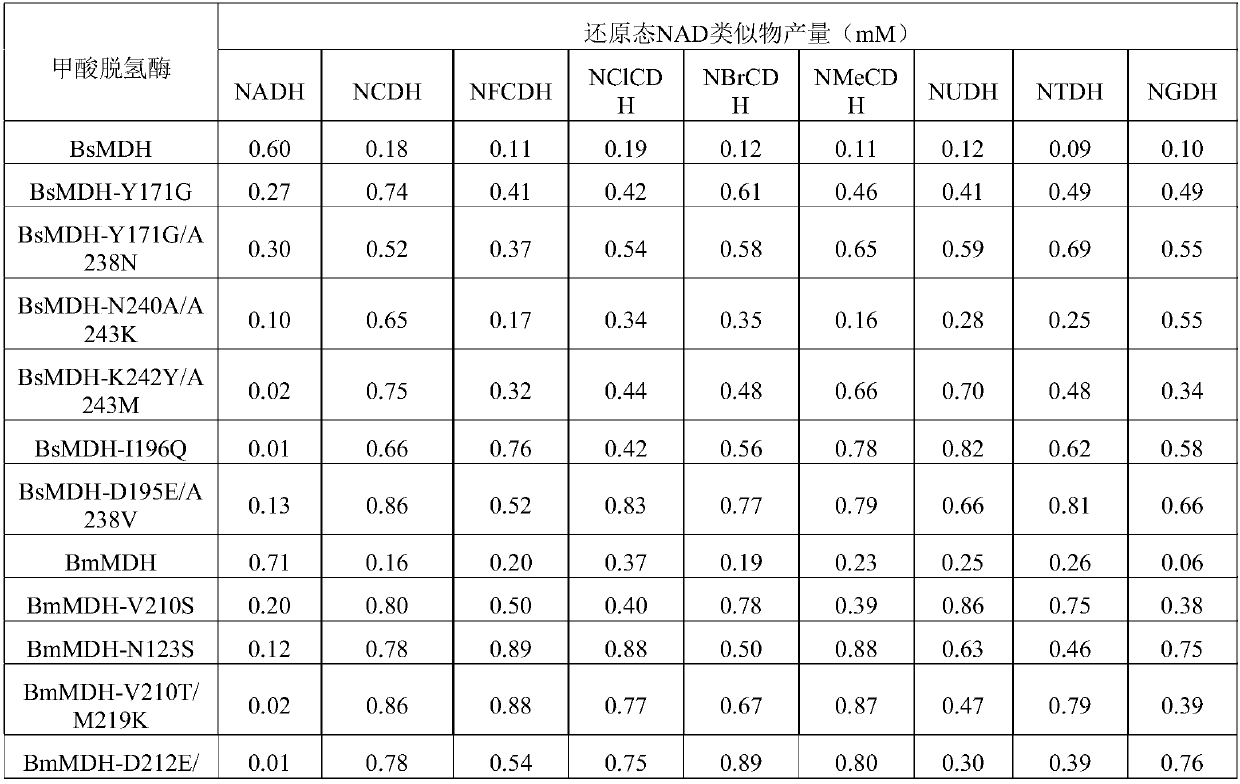
[0032] NAD and its analogs NCD, NFCD, NBrCD, NClCD, NMeCD, NGD, NTD or NUD, and methanol dehydrogenase BsMDH, BsMDH-Y171G, BsMDH-Y171G / A238N, BsMDH-N240A / A243K, BsMDH-K242Y / A243M , BsMDH-I196Q, BsMDH-D195E / A238V, BmMDH, BmMDH-V210S, BmMDH-N123S, BmMDH-V210T / M219K, BmMDH-D212E / M219R, BmMDH-Q217E, BmMDH-D153T / V210S were de-NAD analog-methanol one by one Hydrogenase combination, react according to the following method: dissolve 1mM NAD or similar, 4mM methanol and 80μg methanol dehydrogenase in 1mL HEPES buffer with a concentration of 50mM and pH 7.5, mix well, and react at 30°C for 20min, Take 20 μL for analysis.
[0033] According to the analysis method of Comparative Example 1, it was found that all the samples used had characteristic absorption peaks at 340 nm, but the intensity of absorption peaks obtained by different combinations was significantly differen...
Embodiment 2
[0038] Example 2: Preparation of reduced NAD analogs
[0039] The reaction system in Example 1 is scaled up and can be used to prepare reduced NAD analogs. Take NUDH as an example to illustrate the preparation process. 20mM NUD, 25mM methanol and 5mg methanol dehydrogenase BsMDH-Y171G were dissolved in 10mL sodium phosphate buffer solution with a concentration of 50mM and a pH of 5.7, mixed well, and reacted at 30°C for 80min. Freeze-dry directly after the reaction, concentrate to a total volume of about 4 mL, separate with a formic acid-type anion exchange resin column, track and collect the product at an ultraviolet wavelength of 340 nm, and freeze-dry to obtain 5.6 mg of a white powder with a yield of about 44%.
[0040] The above-mentioned white powder sample was subjected to high-resolution mass spectrometry analysis to measure the precise molecular weight (M+H) + It is 643.1026, and the theoretical molecular weight of NUDH (C 20 h 29 N 4 o 16 P 2 + , 643.1054) co...
Embodiment 3
[0042] Example 3: Using methanol as a reducing agent, methanol dehydrogenase catalyzes the reduction of NAD analogs
[0043] Dissolve 0.1mM NBrCD, 0.4mM methanol and 80μg methanol dehydrogenase BsMDH-Y171G / A238N in 1mL PIPES buffer with a concentration of 50mM and pH 8.0, mix well, react at 40°C for 3min, and take 20μL for analysis.
[0044] Analysis by the method of Comparative Example 1 found that the sample had a characteristic absorption peak at 340nm. The concentration of generated NBrCDH reached 60 μM, that is, the yield reached 60%.
[0045] The results of Example 1 and Example 3 show that methanol dehydrogenase can reduce NAD analogues by using methanol as a reducing agent.
[0046] According to the method of Example 3, the same amount of NBrCD, sodium phosphite and phosphite dehydrogenase rsPDH-I151R was used to produce NAD analogues, and the concentration of generated NBrCDH reached 43 μM, that is, the yield reached 43%. It shows that both methanol dehydrogenase Bs...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com