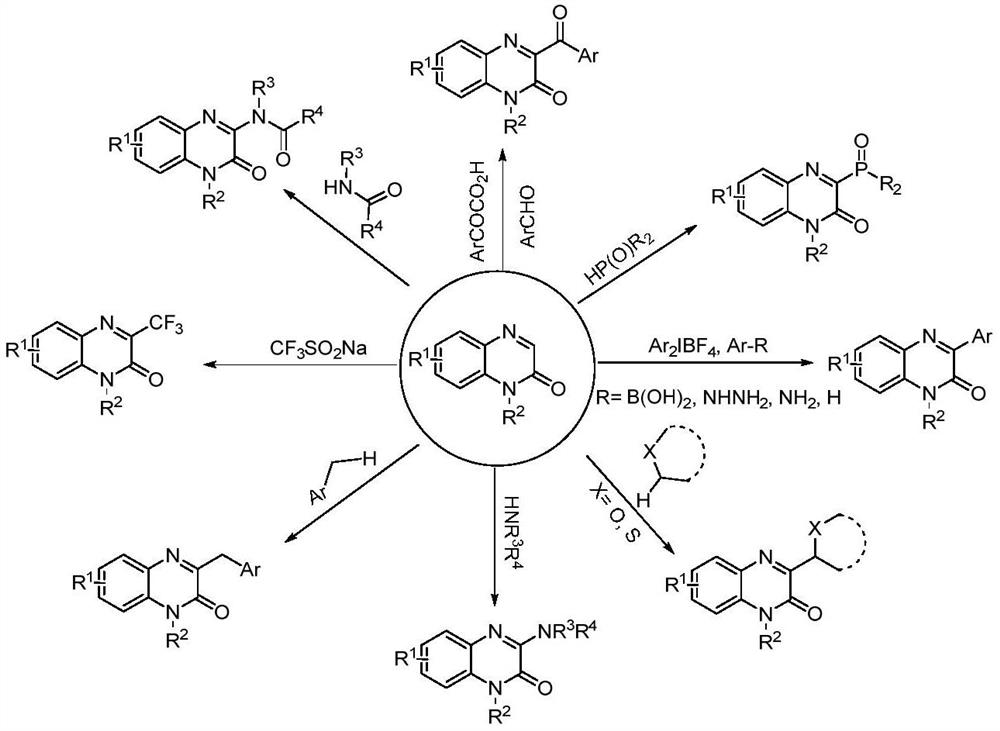
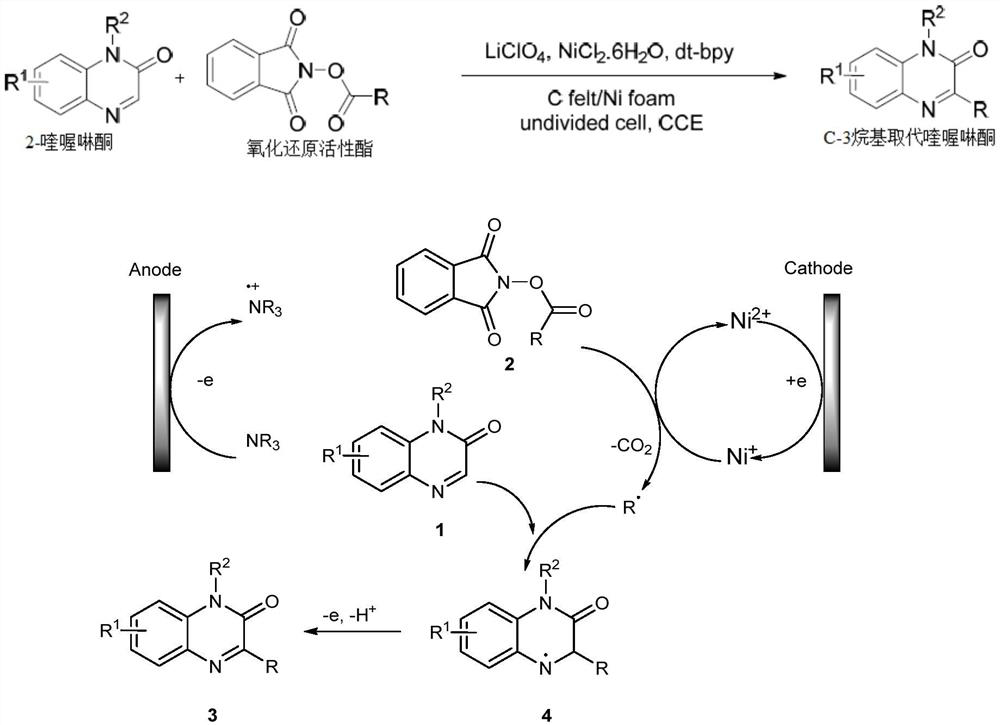
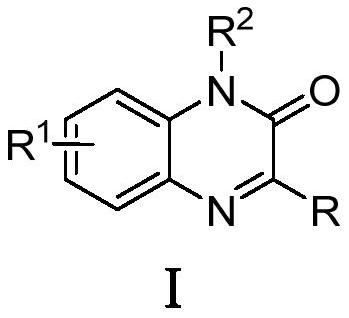
Synthesis method of C-3 alkyl substituted quinoxalinones catalyzed by nickel under electrochemical conditions
The technology of a quinoxalinone and a synthesis method is applied in the field of compound preparation, can solve the problems of high reaction temperature, environmental pollution, low yield and the like, and achieves the effects of simple operation, reduced energy consumption and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]Example 1: Electrochemical method Synthesis 3-cyclohexylquinoline-2 (1H)-ketone
[0024]In a 10 ml single-chamber electrolytic cell, a raw material 2-quinoxalin (0.3 mmol), redox activated ester (0.6 mmol), Liclo4(1.0mmol), NICL2.6h2O (0.6 mmol), 4,4'-di-tert-butyl-2,2'-bisin (0.6 mmol). The sealing device and the argon injection into the tube (three times). Then, N, N-dimethylacetamide (DMA, 4.0 mL) and triethylamine (0.25 mL) were added via a syringe, and the argon balloon was inserted into the bottle with rubber plug. The mixture was first reacted with a magnetic force of 60 ° C for 30 minutes, then at 8 mA / cm2Electrolysis is 3 hours under current density. After the reaction was completed, the mixture was quenched with water and extracted with ethyl acetate (3 × 10 mL). The organic phase is concentrated on a rotary evaporator. The desired product was separated from the column chromatography eluting elut elut elut elut elut Rativity: 87%.
[0025]
[0026]White solid;1H NMR (400MHz...
Embodiment 2
[0027]Example 2: Electrochemical method synthesis 3- (4-isopropylcyclohexyl) quinoxaline-2 (1H)-ketone
[0028]In a 10 ml single-chamber electrolytic cell, a raw material 2-quinoxalin (0.3 mmol), redox activated ester (0.6 mmol), Liclo4(1.0mmol), NICL2.6h2O (0.6 mmol), 4,4'-di-tert-butyl-2,2'-bisin (0.6 mmol). The sealing device and the argon injection into the tube (three times). Then, N, N-dimethylacetamide (DMA, 4.0 mL) and triethylamine (0.25 mL) were added via a syringe, and the argon balloon was inserted into the bottle with rubber plug. The mixture was first reacted with a magnetic force of 60 ° C for 30 minutes, then at 8 mA / cm2Electrolysis is 3 hours under current density. After the reaction was completed, the mixture was quenched with water and extracted with ethyl acetate (3 × 10 mL). The organic phase is concentrated on a rotary evaporator. The desired product was separated from the column chromatography eluting elut elut elut elut elut elut Rativity: 79%.
[0029]
[0030]Whit...
Embodiment 3
[0031]Example 3: Electrochemical Method Synthesis 3- (4, 4-difluorocyclohexyl) quinoxalin-2 (1H)-ketone
[0032]In a 10 ml single-chamber electrolytic cell, a raw material 2-quinoxalin (0.3 mmol), redox activated ester (0.6 mmol), Liclo4(1.0mmol), NICL2.6h2O (0.6 mmol), 4,4'-di-tert-butyl-2,2'-bisin (0.6 mmol). The sealing device and the argon injection into the tube (three times). Then, N, N-dimethylacetamide (DMA, 4.0 mL) and triethylamine (0.25 mL) were added via a syringe, and the argon balloon was inserted into the bottle with rubber plug. The mixture was first reacted with a magnetic force of 60 ° C for 30 minutes, then at 8 mA / cm2Electrolysis is 3 hours under current density. After the reaction was completed, the mixture was quenched with water and extracted with ethyl acetate (3 × 10 mL). The organic phase is concentrated on a rotary evaporator. The desired product was separated from column chromatography eluting elut elut elut elut elut elut elut Yield: 69%.
[0033]
[0034]White...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com