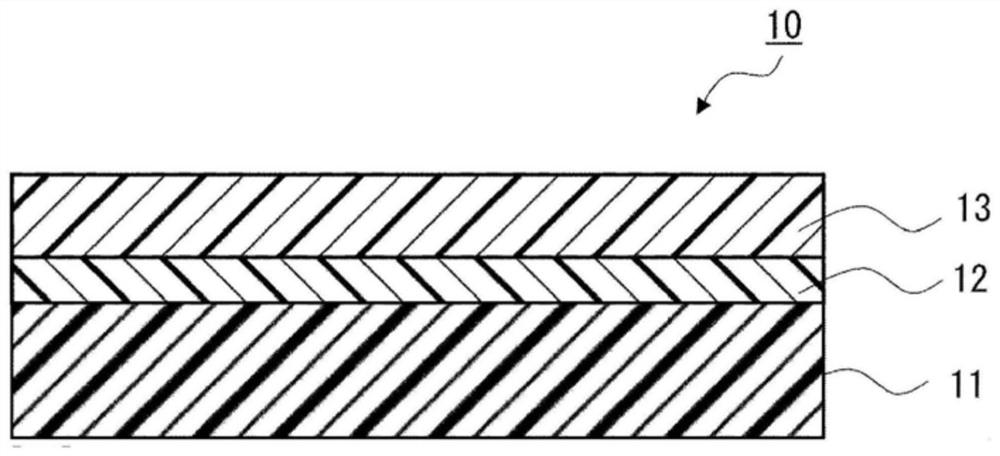
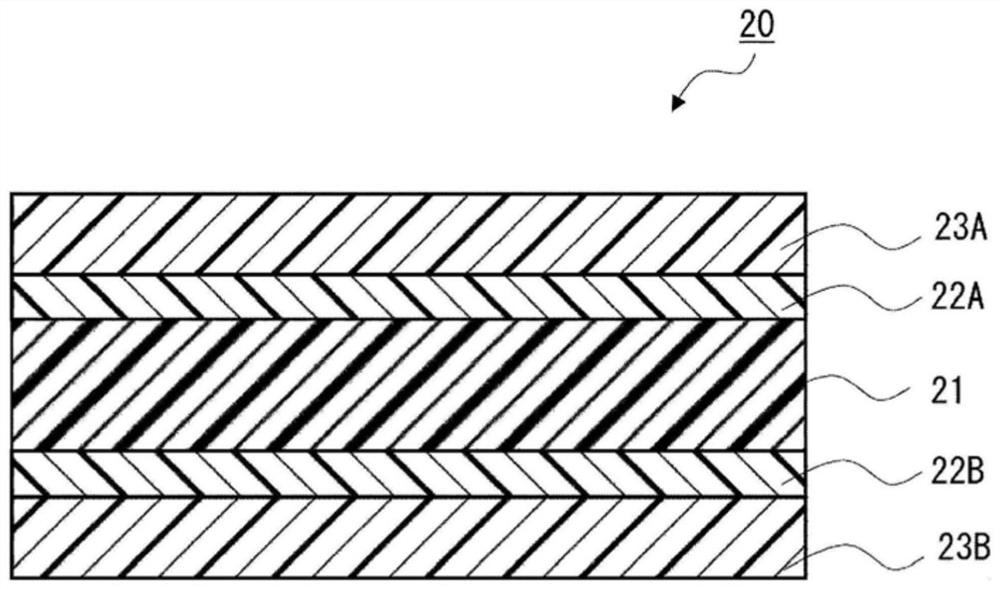
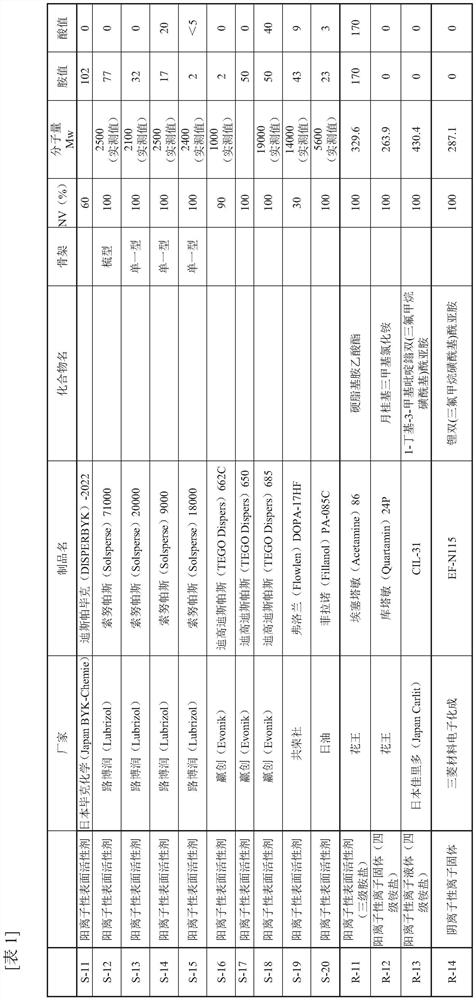
Urethane-based adhesives and adhesive sheets
A technology of urethane and adhesive, applied in the direction of polyurea/polyurethane adhesive, adhesive, adhesive type, etc., can solve problems such as poor re-peelability, reduced re-peelability, and bad conditions, and achieve good Good re-peelability and curability, and the effect of reducing adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0238] Hereinafter, synthesis examples, examples of the present invention, and comparative examples will be described. In addition, in the following description, unless otherwise specified, "part" means a mass part, "%" means a mass %, and "RH" means a relative humidity.
[0239] [Measurement of Mn and Mw]
[0240] The number average molecular weight (Mn) and weight average molecular weight (Mw) are measured by the gel permeation chromatography (GPC) method. The measurement conditions are as follows. In addition, both Mn and Mw are values in terms of polystyrene.
[0241]
[0242] Device: Shimadzu Prominence (SHIMADZU Prominence) (manufactured by Shimadzu Corporation),
[0243] Column: Two LF-804 manufactured by Shodex are connected in series,
[0244] Detector: Differential Refractive Index Detector (RID-10A),
[0245] Solvent: tetrahydrofuran (THF),
[0246] Flow rate: 1mL / min,
[0247] Solvent temperature: 40°C,
[0248] Sample concentration: 0.2%,
[0249] Sam...
Synthetic example 1
[0287] (Synthesis example 1) (1-stage polymerization method)
[0288] 5 parts by mass of active hydrogen group-containing compound (HX-11) and 95 parts by mass of active hydrogen group-containing compound (HX-18) were put into a four-necked flask equipped with a stirrer, a reflux cooling pipe, a nitrogen gas introduction pipe, a thermometer, and a dropping funnel. Parts by mass, 1.6 parts by mass of polyisocyanate (N-11), 67 parts by mass of toluene, 0.020 parts by mass of dioctyltin dilaurate as a catalyst, and 0.008 parts by mass of tin 2-ethylhexanoate were mixed. The temperature of the internal solution was gradually raised to 75° C., and the reaction was performed for 3 hours.
[0289] The ratio of the number of moles of isocyanate groups in the polyisocyanate (N) used in the reaction to the total number of moles of active hydrogen groups in all active hydrogen group-containing compounds (HX) used in the reaction (NCO / H ratio) was 0.49.
[0290] After the disappearance ...
Synthetic example 13
[0296] (Synthesis Example 13) (Multistage Polymerization Method)
[0297] 100 parts by mass of compound (HX-13) containing active hydrogen group, 17 parts by mass of polyisocyanate (N-13), toluene 29.3 parts by mass and 0.01 part by mass of dioctyltin dilaurate as a catalyst were mixed. The temperature of the internal solution was gradually raised to 100° C., and the reaction was performed for 2 hours. Then, after cooling to 25 degreeC and adding 90.7 mass parts of ethyl acetates and 0.5 mass parts of acetylacetones, 3.4 mass parts of active hydrogen group containing compounds (HX-21) were dripped over 1 hour. After maintaining the liquid temperature at 25°C and continuing the reaction for 1 hour, 1.2 parts by mass of an active hydrogen group-containing compound (HX-22) was added and reacted. After the disappearance of the remaining isocyanate groups was confirmed by infrared spectroscopic analysis (IR analysis), the reaction was terminated.
[0298] In this way, a colorles...
PUM
| Property | Measurement | Unit |
|---|---|---|
| amine value | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| gel fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com