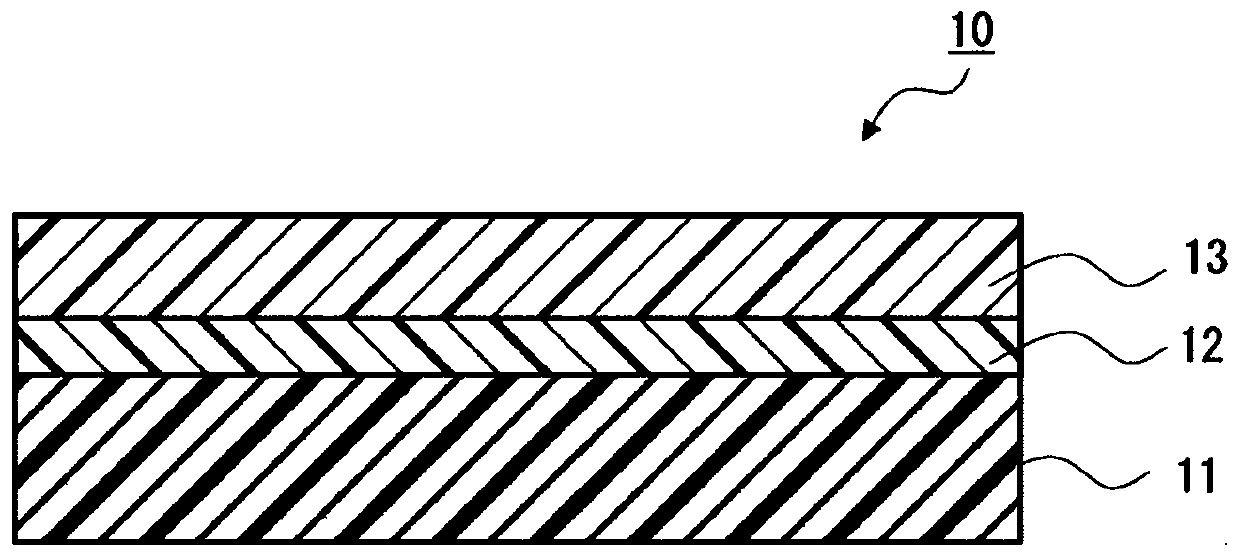
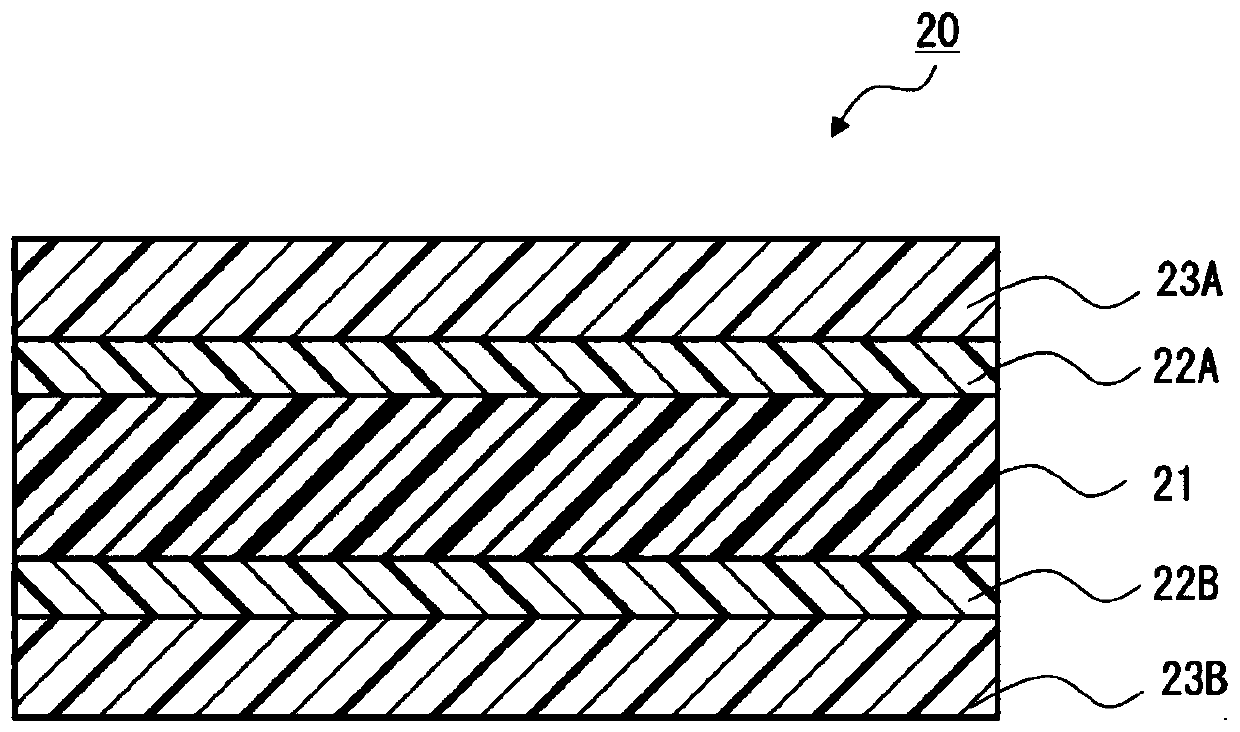
Pressure-sensitive adhesive, pressure-sensitive adhesive sheet, and method for manufacturing hydroxyl-terminated urethane prepolymer
A technology of urethane and prepolymer, applied in the direction of adhesives, adhesive types, polyurea/polyurethane adhesives, etc., can solve problems such as bad conditions, poor re-peelability, pollution, etc., and reach the pot life Good, good initial hardening effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0214] Hereinafter, synthesis examples, examples of the present invention, and comparative examples will be described. In addition, in the following description, unless otherwise indicated, "part" means a mass part, "%" means a mass %, and "RH" means a relative humidity.
[0215] [Measurement of Mn and Mw]
[0216] The number average molecular weight (Mn) and weight average molecular weight (Mw) are measured by the gel permeation chromatography (GPC) method. The measurement conditions are as follows. In addition, both Mn and Mw are values in terms of polystyrene.
[0217]
[0218] Device: Shimadzu Prominence (SHIMADZU Prominence) (manufactured by Shimadzu Corporation),
[0219] Pipe string; two TSKgel GMH manufactured by TOSOH are connected in series,
[0220] Detector: Differential Refractive Index Detector (RID-10A),
[0221] Solvent: tetrahydrofuran (THF),
[0222] Flow rate: 1mL / min,
[0223] Solvent temperature: 40°C,
[0224] Sample concentration: 0.1%,
[0...
Synthetic example 1
[0272] (Synthesis example 1) (two-stage polymerization method)
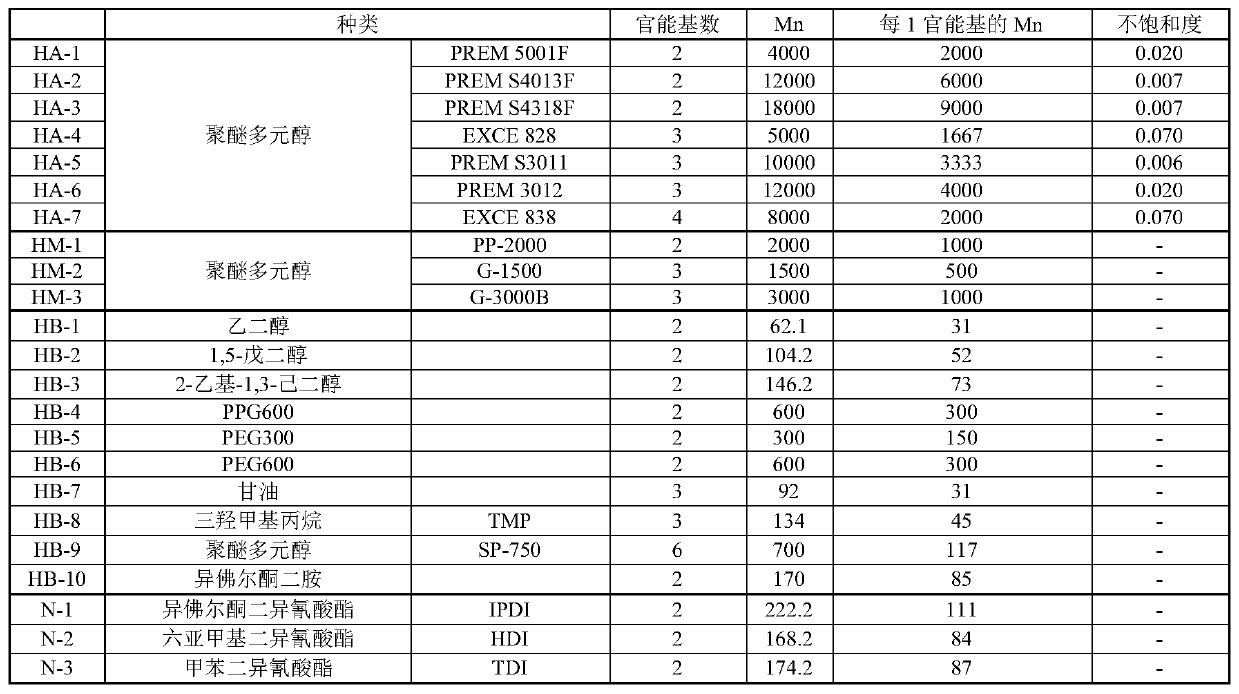
[0273] 100 mass parts of polyether polyol (HA-1), 10 mass parts of polyisocyanate (N-1), 47 Parts by mass of toluene and 0.01 part by mass of dioctyltin dilaurate as a catalyst were mixed. The temperature of the content liquid was gradually raised to 80° C., and the reaction was performed for 2 hours to obtain an isocyanate group-terminated urethane prepolymer (reaction of the first stage). Next, after cooling the content liquid to 60 degreeC and adding 28 mass parts of ethyl acetate, 4 mass parts of active hydrogen group containing compounds (HB-2) were added and made to react (reaction of the 2nd stage). In the whole reaction of the first stage and the second stage, the number of moles of the isocyanate group (NCO) of the polyisocyanate (N) used in the reaction is relative to the total active hydrogen group-containing compound (HX) used in the reaction. ) (in the above example, the ratio (NCO / H) of the total ...
Synthetic example 43
[0279] (Synthesis Example 43) (one-stage polymerization method)
[0280] 100 mass parts of polyether polyol (HA-6), 4 mass parts of active hydrogen-containing compounds (HB- 2) 47 parts by mass of toluene and 0.01 part by mass of dioctyltin dilaurate as a catalyst were mixed and the temperature was gradually raised to 80°C. 9 parts by mass of polyisocyanate (N-1) and 28 parts by mass of ethyl acetate were added to the content liquid, and reacted for 2 hours. The molar number of the isocyanate group (NCO) that the polyisocyanate (N) used in the reaction has is relative to the compound (HX) (HX) of all active hydrogen groups used in the reaction (being (HA-6) and The ratio (NCO / H) of the total number of moles of active hydrogen groups (H) contained in (HB-2)) was 0.58.
[0281] After confirming the disappearance of the remaining isocyanate groups by infrared spectroscopic analysis (IR analysis), the content liquid was cooled to complete the reaction to obtain a white opaque hy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of unsaturation | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| degree of unsaturation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com