Butenolide dimer having effects of restraining COX-2 and resisting oxidation and application of butenolide dimer having effects of restraining COX-2 and resisting oxidation
A dimer and butyrolactone technology, applied in organic chemistry, medical preparations containing active ingredients, anti-inflammatory agents, etc., can solve problems such as kidney adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Isolation, purification and identification of strains.
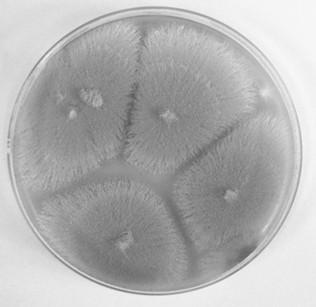
[0026] Bacteria were isolated and purified from the sediments of the Mangrove Reserve in Techeng Island, Zhanjiang Aspergillus terreus SCAU011, its typical culture pictures are as follows figure 2 Shown: the colony is round, the hyphae are white, and the spores are khaki. After ITS rDNA detection, compared with Gene Bank data, identified as Aspergillus terreus fungus.
Embodiment 2
[0027] Example 2 Preparation of fermented product.
[0028] Preparation method of seed medium: Potato extract 200 mL, glucose 20 g, sea salt 30 g, dilute to 1 L with water. Put the PDB medium into twenty 500 mL Erlenmeyer flasks, about 150 mL each, sterilize at 121 °C for 25 minutes by high-pressure steam, and set aside.
[0029] Rice medium configuration method: 80 g of commercially available rice, 0.4 g of yeast extract, 0.4 g of glucose, 120 mL of sterile water, and 3.6 g of sea salt were placed in 1 L Erlenmeyer flasks, a total of 60 bottles. Autoclave at 121°C for 25 minutes and set aside.
[0030] Pick an appropriate amount of fungi with a sterile bamboo stick Aspergillus terreus The SCAU011 strain was inoculated into the seed medium, and cultured on a shaker (180 rpm) at 28°C for 3 days to obtain the seed liquid, and then inoculated 10 mL of the seed liquid into a 1 L Erlenmeyer flask containing rice medium with a pipette gun, and then After static culture at 28° C....
Embodiment 3
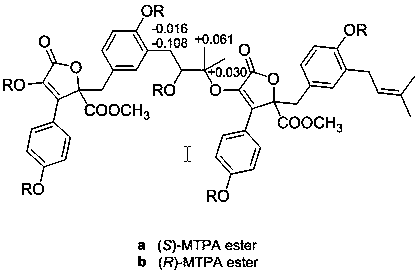
[0032] Example 3 Preparation of aromatic croton lactone dimer.
[0033] According to the method of Example 2, 4.0 kg of fermented rice medium was obtained, the rice medium was soaked with 95% ethanol, the ethanol was recovered from the leaching solution, and the remaining water phase was extracted with ethyl acetate, concentrated under reduced pressure to obtain the ethyl acetate extract 60g. The ethyl acetate extract was subjected to column chromatography on normal-phase silica gel (100-200 mesh), using dichloromethane-methanol as the eluent, and gradient elution was performed from a volume ratio of 100:0 to 0:100, according to TLC The fractions were combined according to the analysis situation, and the elution solvent was recovered to obtain 7 fractions (Fr.1-Fr.7).
[0034] Fr.5 further carried out normal-phase silica gel (200-300 mesh) column chromatography with dichloromethane-acetone as the eluent, combined the fractions according to the thin-layer chromatography, recov...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap