Catalyst and its preparation method and application, cyclic carbon dioxide-based polycarbonate and its preparation method
A technology based on polycarbonate and carbon dioxide, applied in chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problems of unstable polyanhydride structures and pending development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] The present invention provides the preparation method of the catalyst for synthesizing cyclic carbon dioxide-based polycarbonate described in the above technical scheme, comprising the following steps:
[0057] Mixing 4,4-diaminodiphenylmethane, acetylacetone, p-toluenesulfonic acid and toluene, and performing the first hydroxylamine condensation reaction to obtain the first ligand;
[0058] mixing the ethanol solution of the first ligand, the hydrochloric acid solution of 1,4-dioxane, and 4,4-diaminodiphenylmethane to perform a second hydroxylamine condensation reaction to obtain the second ligand;
[0059] Mixing the solution of the second ligand and the solution of the zinc compound to perform a substitution reaction to obtain a catalyst for synthesizing cyclic carbon dioxide-based polycarbonate;
[0060] The zinc compound in the zinc compound solution includes zinc compounds of alkyl zinc or alkyl derivatives.
[0061] In the present invention, unless otherwise spe...
Embodiment 1
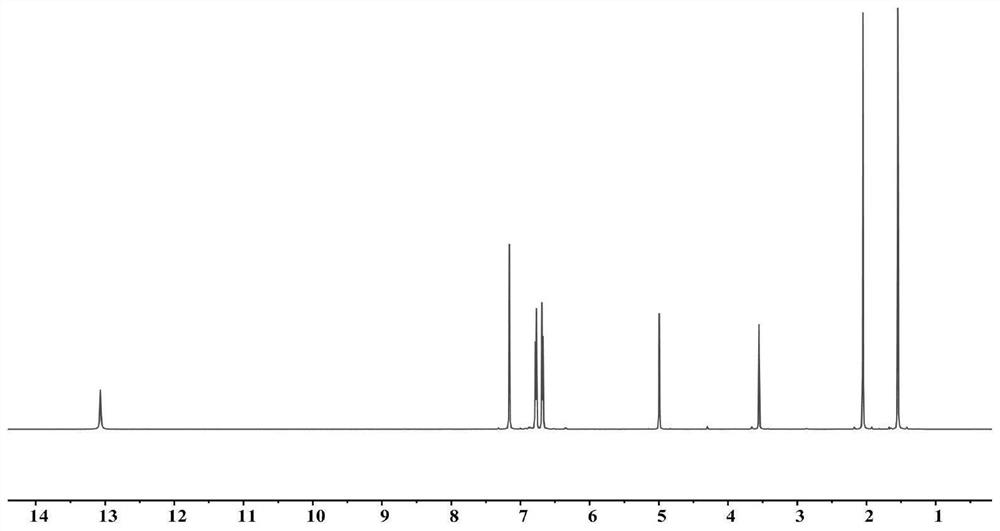
[0085] 4,4-Diaminodiphenylmethane (11.6g, 58.4mmol, 1.0eq), acetylacetone (12mL, 117mmol, 2.0eq) and p-toluenesulfonic acid (1.0g, 5.8mmol, 0.01eq) were dissolved in 1L of toluene Reflux at 130°C for 2h, carry out the first hydroxylamine condensation reaction, use saturated NaHCO 3 The resulting mixture was washed with an aqueous solution, and the solvent was removed by rotary evaporation, and 19 g of a khaki solid was obtained after washing with hexane, which was the first ligand with a yield of 90%; the H NMR spectrum of the first ligand (500 MHz 1 H NMR benzene-d 6 )See figure 1 ; NMR data: δ13.07(s, 2H, -OH), δ6.77-6.79(d, J=10.0Hz, 4H, C-H), δ6.67-6.69(d, J=10.0Hz, 4H, C-H), δ5.00(s, 2H, C-H), δ3.55(s, 2H, CH 2 ),δ2.05(s,6H,CH 3 ), δ1.55(s,6H,CH 3 ), according to the nuclear magnetic spectrum information, the structure of the compound is the above-mentioned target first ligand structure;
[0086] The first ligand (3g, 8.3mmol, 1.0eq) was dissolved in 120mL ethanol, ...
Embodiment 2
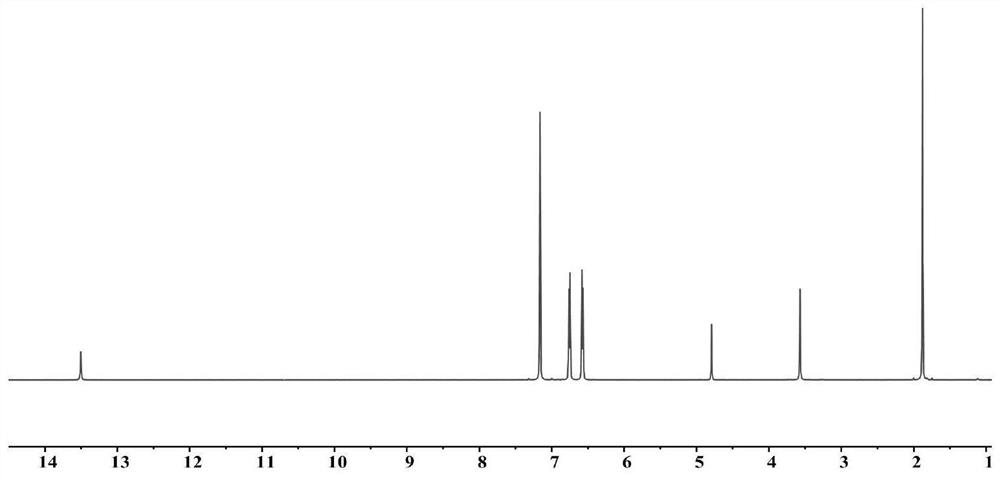
[0089] 4,4-Diaminodiphenylmethane (24.0g, 117.0mmol, 2.0eq), acetylacetone (12mL, 117mmol, 2.0eq) and p-toluenesulfonic acid (100.0g, 580.0mmol, 1.0eq) were dissolved in 1L of toluene Reflux at 100°C for 2 hours to carry out the first hydroxylamine condensation reaction, using saturated NaHCO 3The resulting mixture was washed with an aqueous solution, and the solvent was removed by rotary evaporation, and 20.1 g of a khaki solid was obtained after washing with hexane, which was the first ligand with a yield of 95%; the H NMR spectrum of the first ligand (500 MHz 1 H NMR benzene-d 6 )see picture 1;
[0090] The first ligand (3g, 8.3mmol, 1.0eq) was dissolved in 250mL ethanol, and the HCl solution of dioxane (the concentration of hydrochloric acid was 4.0M, 8.4mL, 33.6mmol, 4.0eq) was added dropwise, and then 4,4-Diaminodiphenylmethane (1.65g, 8.3mmol, 1.0eq), reflux overnight at 90°C for the second hydroxylamine condensation reaction, disperse the resulting solid in water, ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com