A kind of alternate copolymerization method for preparing fluoropolymer based on dibromo compound and diene monomer
A double-brominated compound and alternating copolymerization technology is applied in the field of alternating copolymerization of fluorine-containing polymers prepared based on double-brominated compounds and diene monomers, and achieves the effects of expanding the scope of application, high practical application value, and high research value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
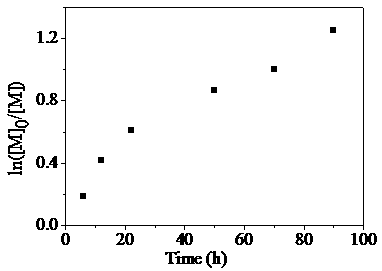
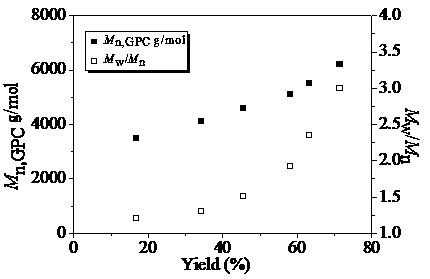
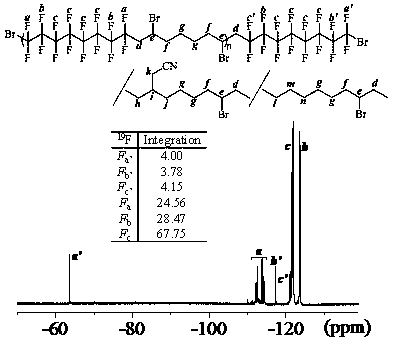
[0031] Alternate Copolymerization of Dibromo Compounds and Non-conjugated Dienes via Free Radical Stepwise Transfer-Addition-Termination Polymerization in Mixed Solvent System of Dimethyl Carbonate and Acetonitrile
[0032] according to n A1 :n B1 :n fac-Ir(ppy)3 :n TBABr = 100:100:2:50 initial molar ratio, difunctional monomer A1 (1,8-dibromohexadecafluorooctane) (0.2799 g, 0.5 mmol), B1 (1,7-octadiene) (74.0 μL, 0.5 mmol), fac -Ir(ppy) 3(6.6 mg, 0.01mmol), TBABr (80.6 mg, 0.25 mmol) was added to the mixed solvent system of acetonitrile and dimethyl carbonate (V 乙腈 :V 碳酸二甲酯 =1:3), add a stirring bar, and seal the tube directly after four deoxygenation operations. Then transfer the ampoule to a magnetic stirrer with a set speed (1500 rpm) and carry out photopolymerization at room temperature under LED blue light illumination. After reaching the given time (20 h), take out and open the airtight container, and use the polymerization system to Tetrahydrofuran was dilute...
Embodiment 2
[0034] Influence of different bases as accelerators on the stepwise transfer-addition-termination polymerization of dibromo compounds and non-conjugated diene radicals in a mixed solvent system of dimethyl carbonate and acetonitrile
[0035] because fac -Ir(ppy) 3 Compared to Ru(bpy) 3 Cl 2 It has more excellent redox stability and longer excited state lifetime, so we first locked the metal photocatalyst as fac -Ir(ppy) 3 . Through literature research, we can know that the addition of alkali compounds will affect the excitation potential when the photocatalyst forms an excited state. Therefore, we add various base compounds to the current polymerization system to investigate the effect of base compounds as accelerators on the gradual transfer-addition-termination of polymerization of dibromo compounds and non-conjugated diene radicals. The results are shown in the following table 1.
[0036] Table 1. Effects of different bases on the stepwise transfer-addition-termina...
Embodiment 3
[0041] In the mixed solvent system of dimethyl carbonate and acetonitrile, the effects of different Lewis acids as accelerators on the stepwise transfer-addition-termination polymerization of dibromo compounds and non-conjugated diene radicals were investigated
[0042] In addition to regulating metal photocatalysts by adding base compounds fac -Ir(ppy) 3 In addition to the excited state redox potential, we also tried to add various Lewis acids to the system to build an oxidation catalytic cycle system to improve the polymerization efficiency. The specific results are shown in Table 2 below.
[0043] Table 2. Effects of different Lewis acids on the stepwise transfer-addition-termination polymerization of dibromocompounds and non-conjugated diene radicals
[0044]
[0045] Polymerization conditions: solution polymerization was carried out under room temperature LED blue light illumination conditions. The feeding ratio is [A1] 0 :[B1] 0 :[ fac -Ir(ppy) 3 ] 0 :[Accele...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com