A kind of oral liquid preparation of desloratadine citrate and preparation method and application thereof
A technology of desloratadine and loratadine, which is applied in the field of pharmaceutical preparations to meet clinical needs, improve compliance, and ensure safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
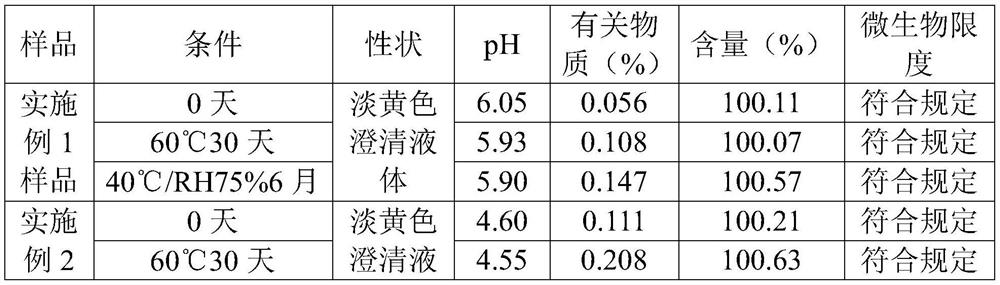
[0046] Embodiment 1 desloratadine citrate oral solution
[0047] Element concentration desloratadine citrate 0.88kg ethyl paraben 0.25kg sucrose 400kg Disodium Edetate 3kg Sodium citrate 2kg apple essence 1.5kg add water to 1000L
[0048] The preparation method of the present embodiment is:
[0049] (1) take by weighing desloratadine citrate, ethyl paraben, sucrose, disodium edetate, sodium citrate, apple essence of recipe quantity;
[0050] (2) adding the ethyl paraben and sucrose of the recipe quantity into an appropriate amount of purified water, stirring and boiling for about 20 minutes, and cooling to room temperature;
[0051] (3) Add desloratadine citrate, disodium edetate, sodium citrate and apple essence to the above solution, stir for about 30 minutes to dissolve, add purified water to 1000L, and fill to obtain the final product.
Embodiment 2
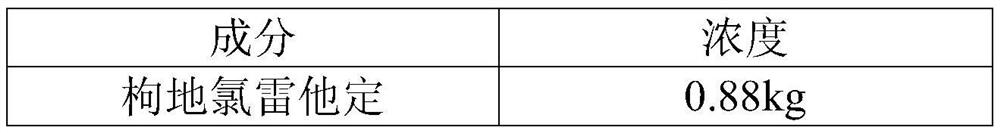
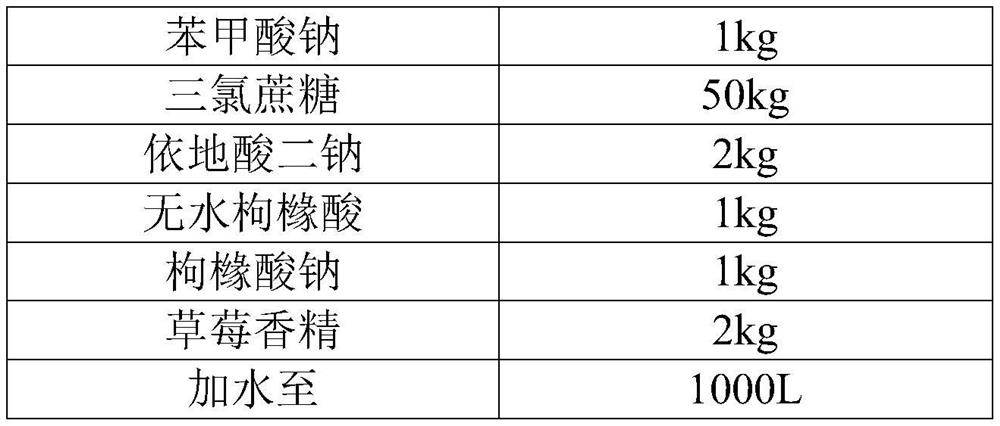
[0052] Embodiment 2 desloratadine citrate oral solution
[0053]
[0054]
[0055] The preparation method of the present embodiment is:
[0056] (1) take by weighing the desloratadine citrate, sodium benzoate, sucralose, disodium edetate, anhydrous citric acid, sodium citrate, strawberry essence of recipe quantity;
[0057] (2) adding the sodium benzoate of the recipe quantity to an appropriate amount of purified water, stirring and dissolving;
[0058] (3) Add desloratadine citrate, sucralose, disodium edetate, anhydrous citric acid, sodium citrate, strawberry essence to the above solution, stir for about 30 minutes to dissolve, and add purified water to 1000L, fill and get it.
Embodiment 3
[0059] Embodiment 3 desloratadine citrate oral solution
[0060] Element concentration desloratadine citrate 0.88kg methylparaben 0.3kg Propylparaben 0.03kg Sorbitol 10kg Xylitol 15kg Sodium dihydrogen phosphate 0.3kg Disodium phosphate 4.0kg Disodium Edetate 1.5kg orange essence 1.0kg Amaranth 0.1kg add water to 1000L
[0061] The preparation method of the present embodiment is:
[0062] (1) take by weighing the desloratadine citrate, methylparaben, propylparaben, sorbitol, xylitol, sodium dihydrogen phosphate, disodium hydrogen phosphate, disodium edetate, orange essence of recipe quantity , Amaranth;
[0063] (2) methylparaben and propylparaben of recipe quantity are added to an appropriate amount of purified water, stirred and boiled to dissolve in 20 minutes, and cooled to room temperature;
[0064] (3) Add desloratadine citrate, sorbitol, xylitol, sodium dihydrogen phosphate, dis...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap