Ethylene-selective electrode with a mixed valence cu4o3catalyst
一种电极、混合物的技术,应用在电极、电极形状/类型、电解过程等方向,能够解决没有得到、无法产生电极等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0157] Cu 3 o 4 The synthesis of the phase was inspired by the synthetic route (mg range) described in the publication of Zhao et al. (Zhao et al., Chem. Mater. 2012, 24, pp. 1136-1142).
[0158] A typical synthesis involves dissolving 50 mM Cu(NO 3 ) 2 ·3H 2 O. The solution was stirred for 10 min and then transferred into a 1.5 L glass liner which was then placed into a stainless steel autoclave (Autoclave BR-1500, Berghof). The autoclave was closed and the reaction mixture was kept inside the autoclave at 130 °C for 24 h. After 24 h, the glass liner and reaction mixture were removed from the autoclave and cooled to room temperature by means of an ice bath. The reaction product precipitated in the glass liner. After cooling, the supernatant was removed from the glass liner, and the remaining solid product was collected by centrifugation and washed three times with ethanol. The powder obtained was first dried in a stream of argon and then in vacuo. Finally, the powder...
example 2
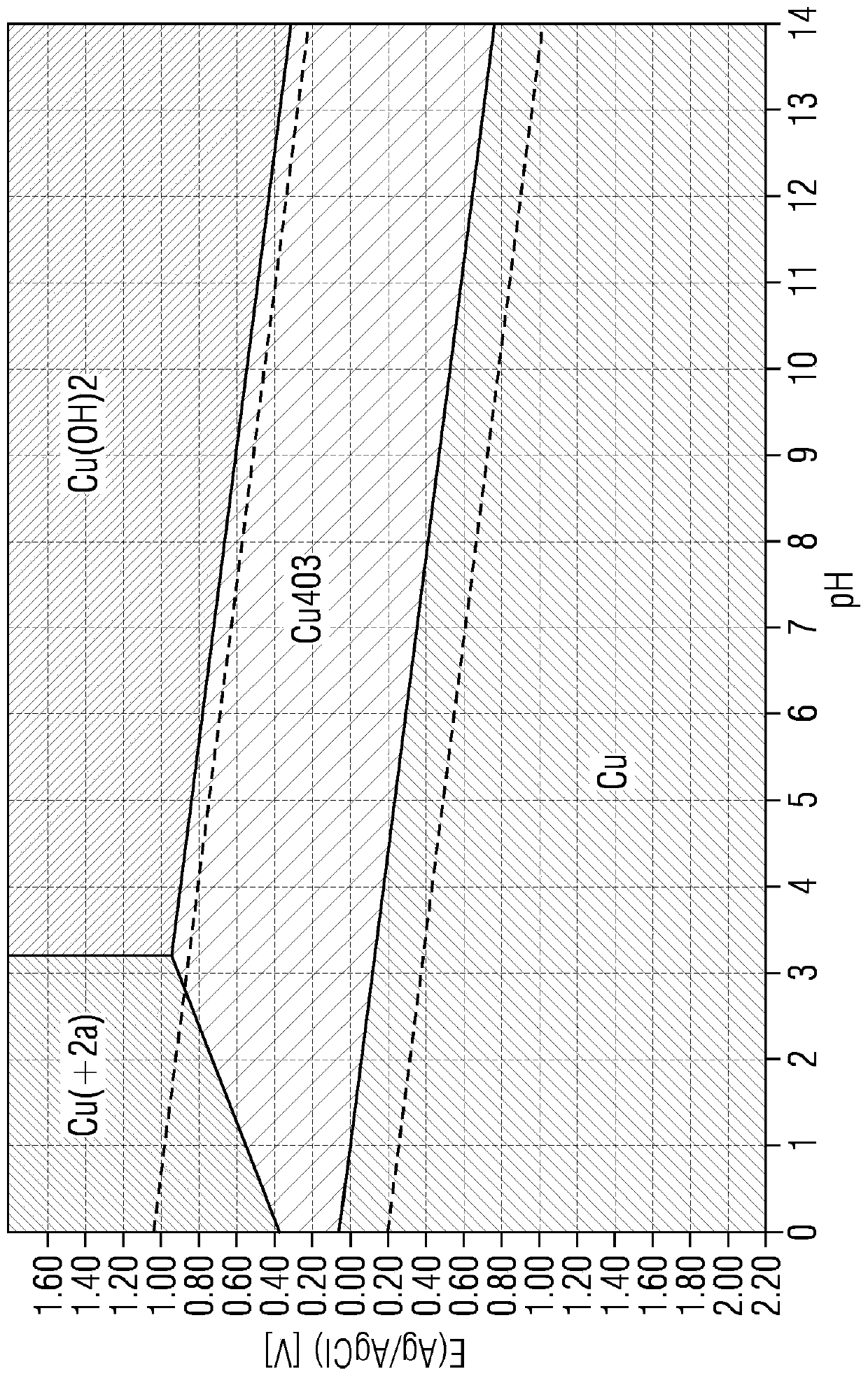
[0167] still based on Figure 17 The double-film test device containing Cu was tested 4 o 3 GDE as catalyst. GDE was prepared as described above. 0.5M H 2 SO 4 Used as electrolyte between the anion exchange membrane AEM (Sustainion x37-50 membrane) and cation exchange membrane CEM (Nafion 117 membrane) and as electrolyte circulating in the chamber behind the anode. Measurements were performed in constant current mode, i.e. the GDE was tested at different constant current values. Use with IrO 2 A coated solid Ti-plate was used as the counter electrode. The electrolytic cell was equipped with an Ag / AgCl / 3M KCl reference electrode. For galvanostatic measurements, the cathode was connected as the working electrode. due to H 2 SO 4 The whole was used as an electrolyte, so the pH was close to zero during the experiment. This experiment shows that in a novel dual-film device with a zero gap (Zero Gap) anode (CEM directly against the anode surface) and a zero gap (Zero Gap...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface density | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com