Aqueous compositions comprising bilastine and mometasone
A composition, water-based technology, applied in the directions of pharmaceutical combinations, non-active ingredients of polymer compounds, medical preparations containing active ingredients, etc., can solve problems such as stable compositions that are not mentioned
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063] Methods for the preparation of these mometasone derivatives are well known in the art (eg, M.B. Smith, J. March, March's Advanced Organic Chemistry, Wiley-Interscience, 5th edition). Likewise, mometasone and mometasone derivatives may be present in the aqueous pharmaceutical composition as a free compound or as a solvate (eg, hydrate, alcoholate, etc.), and both forms are included within the scope of the present invention. Thus, for example, suitable forms of mometasone furoate in the aqueous pharmaceutical compositions of the invention include anhydrous forms or hydrated forms, such as monohydrated forms. In a preferred embodiment, the aqueous pharmaceutical composition of the present invention comprises mometasone hydrate. Solvation methods are well known in the art.
[0064] In a preferred embodiment, based on the total weight of the composition, the amount of mometasone or a pharmaceutically acceptable derivative thereof in the aqueous pharmaceutical composition of...
Embodiment 1
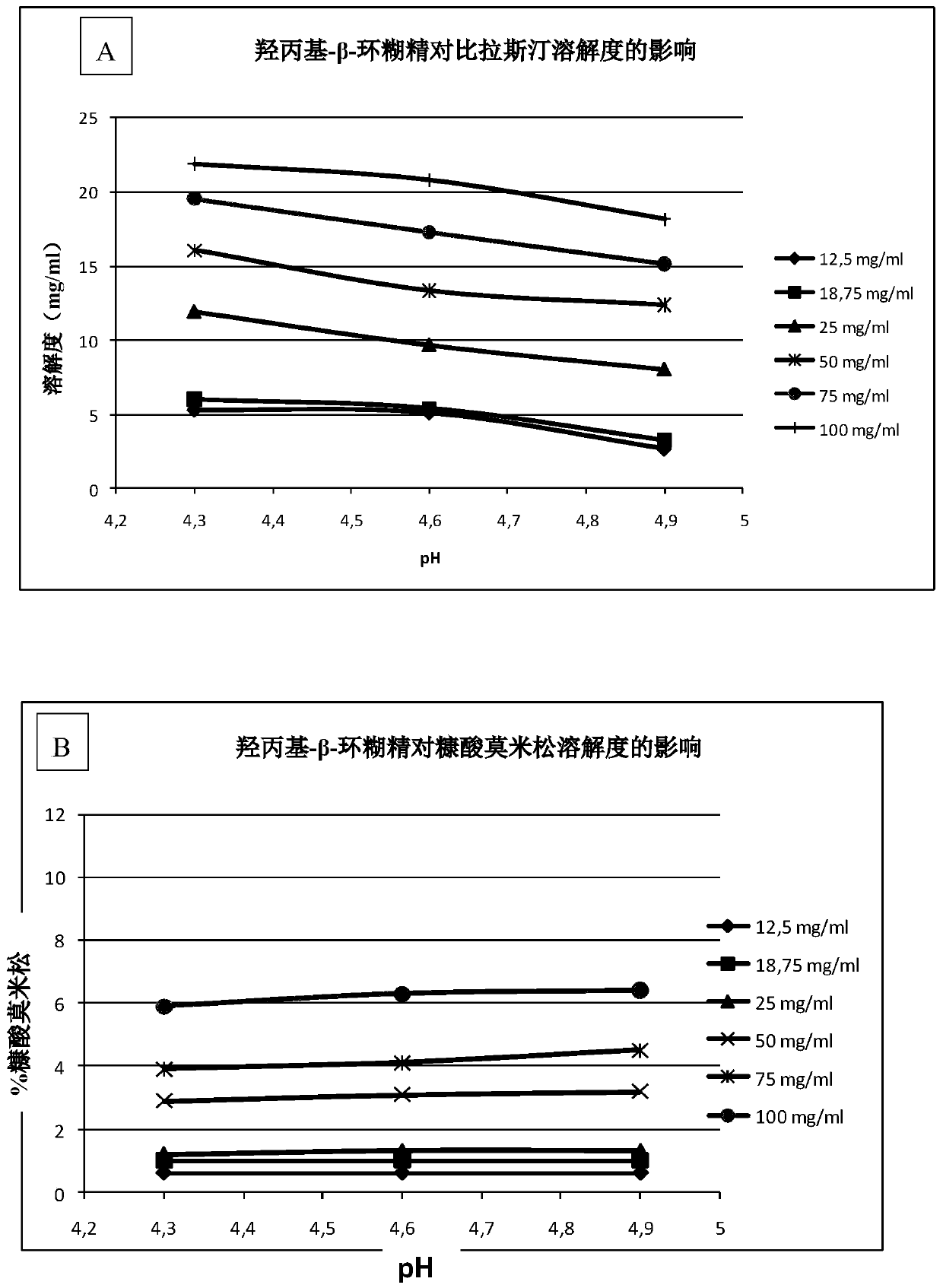
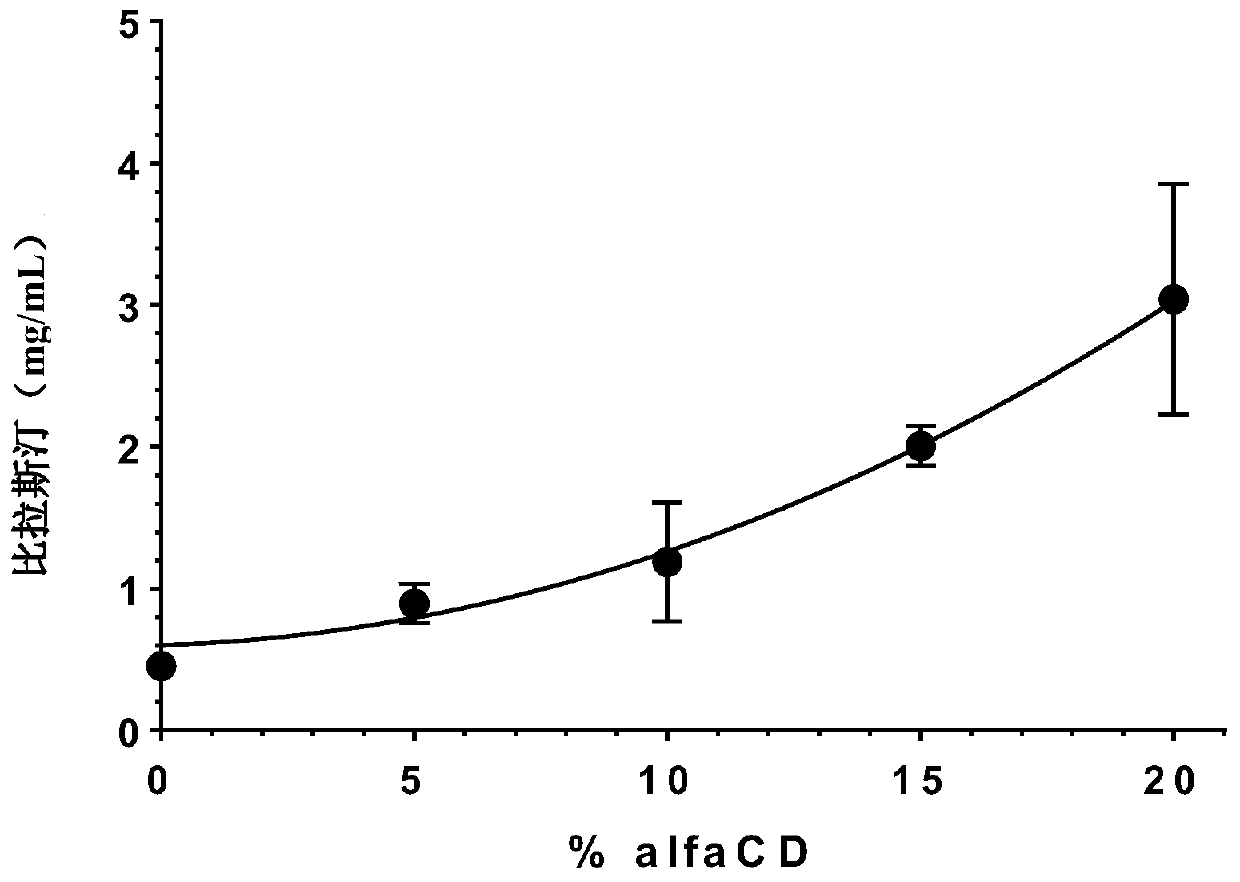
[0171] Example 1-visual evaluation using HPBCD as the uniformity of the solubilizer
[0172] The formulations of this example were prepared as described above. The following table shows the content of each component in the final preparation of the embodiment of the present invention:
[0173]
[0174]
[0175] *0.517 mg / mL mometasone furoate monohydrate is equivalent to 0.5 mg / mL mometasone furoate anhydrous form.
[0176] Two other aqueous pharmaceutical compositions of the present invention were prepared, which had the same composition as the above composition, but changed the content of bilastine to 2 mg / mL and 8 mg / mL.
[0177] pH measurement: 4.6
[0178] Homogeneity was assessed visually: the formulation was homogeneous.
Embodiment 2
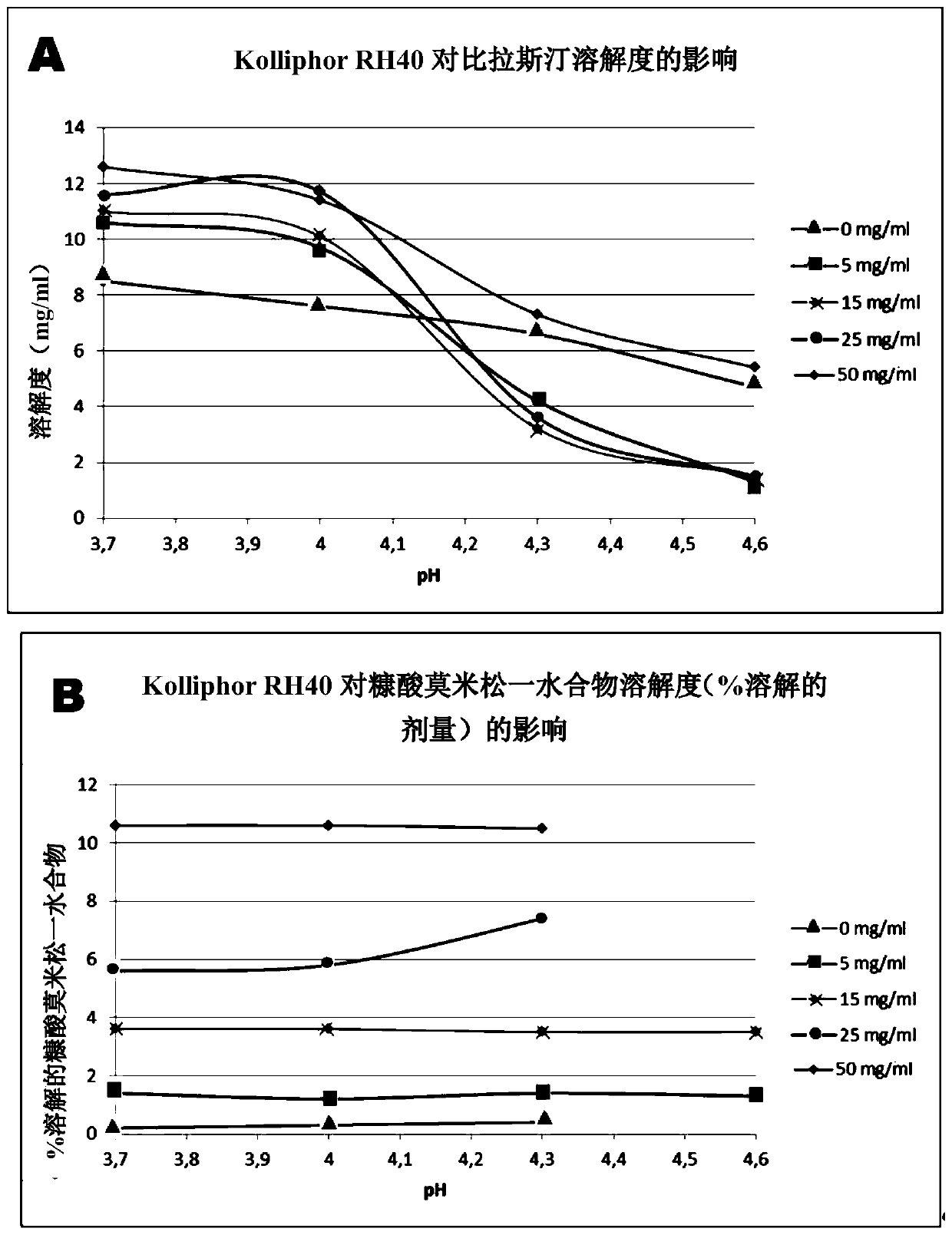
[0179] Example 2 - Comparative. Visual Evaluation of Uniformity of Alternative Solubilizers Using Bilastine and Mometasone
[0180] Five formulations in which cyclodextrin was replaced by alternative solubilizers of bilastine and mometasone were prepared and evaluated for visual homogeneity. The formulation of this example was prepared in a similar manner to that described above, but this time with (8g.), 35 (8g.) (Fagron), 40 (8g.) (Fagron), 80 (8 g.) (Basf) or Poloxamer 188 (10 g.) (Basf) were substituted for cyclodextrins because of their surfactant properties. In the formulation of this example, Spam 80 acts as an antifoaming agent.
[0181] 2.1 Evaluation by visual method uniformity.
[0182] The following table shows the usage in this example Exact amounts of components in the final formulation as solubilizers for bilastine and mometasone:
[0183]
[0184]
[0185] Evaluation of uniformity by visual inspection: There is phase separation and thus the f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com