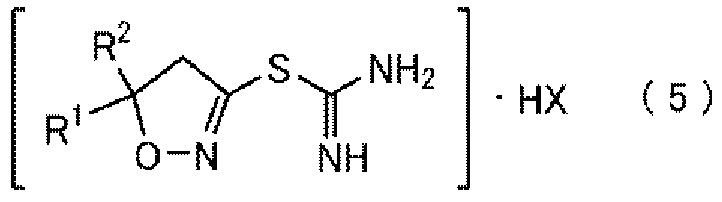
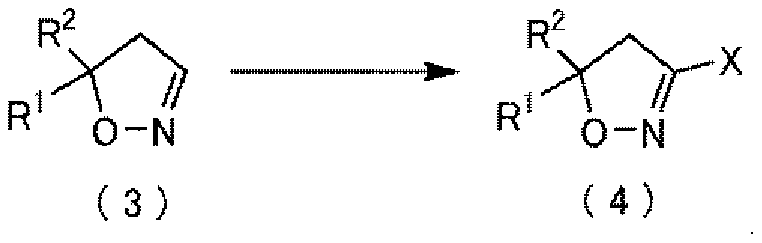
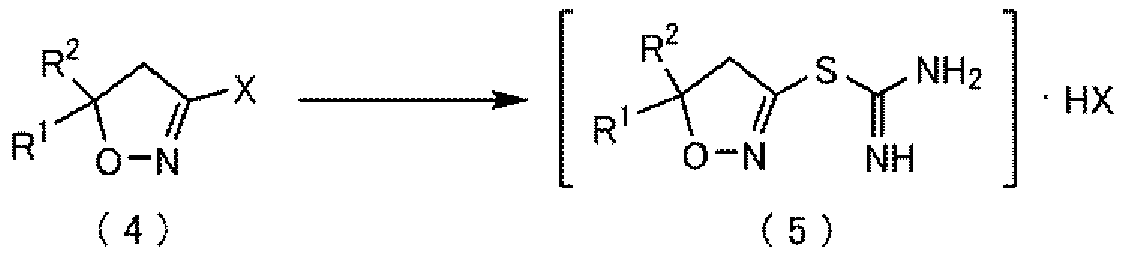
Method for producing thiocarboxamidine salt compound
A technology for manufacturing methods and compounds, which is applied in the field of compounds of the formula, and can solve problems such as uneconomical, uneconomical, and unoptimized methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0274] Production of [5,5-dimethyl(4,5-dihydroisoxazol-3-yl)]thioformamidine hydrochloride (5-a)
[0275] Step (C: Chlorination) and Step (D: Isothiouronium Formation)
[0276] [Chemical 51]
[0277]
[0278] (1) Production of 3-chloro-5,5-dimethyl-4,5-dihydroisoxazole (4-a)
[0279] Process (C: Chlorination)
[0280] 5,5-Dimethyl-4,5-dihydroisoxazole (3-a; 186mg, 1.88mmol, 100mol%) was dissolved in acetonitrile (0.94mL, 0.5L (liter) / mol, with (3- a) as the basis) and water (0.28mL, 0.15L / mol, based on (3-a)). Introduce chlorine gas therein at 25~30°C (as a gas of 50mL, metered with an airtight syringe at 25°C, gas specific gravity 2.935g / L (liter) (25°C), 0.147g, 2.07mmol, 110mol%), Stir at the same temperature for 1 hour. Production of the target 3-chloro-5,5-dimethyl-4,5-dihydroisoxazole (4-a) was confirmed by GC-MS analysis of the reaction mixture. As a result of GC analysis (area percentage) of the reaction mixture, the components other than the solvent etc. in the...
Embodiment 2~ Embodiment 21 and comparative example 1
[0287] Production of 3-chloro-5,5-dimethyl-4,5-dihydroisoxazole (4-a)
[0288] Process (C: Chlorination)
[0289] [Chemical 52]
[0290]
[0291] The chlorination of the process (C) was performed similarly to Example 1 (1) except having changed the solvent as shown in following Table 1. As a result of GC analysis (area percentage) of the reaction mixture, the target product, raw materials, and by-products other than these are shown in Table 1 below. In addition, the results of Example 1(1) are also shown in Table 1.
[0292] [Table 1]
[0293]
[0294] Uniformity ◯: The reaction liquid is uniform.
[0295] Uniformity ×: The reaction solution was separated into two layers.
[0296] The reaction proceeds when only a nitrile solvent is used as the reaction solvent. However by-products are also formed (cf. Example 2, Example 7, Example 16, Example 17 and Example 21). On the other hand, when only water was used as the reaction solvent, the yield was as low as 45% (see ...
Embodiment 22
[0298] Production of 3-chloro-5,5-dimethyl-4,5-dihydroisoxazole (4-a)
[0299] Process (C: Chlorination)
[0300] [Chemical 53]
[0301]
[0302] 5,5-Dimethyl-4,5-dihydroisoxazole (3-a; 5.0 g, 50.4 mmol, 100 mol%) was dissolved in acetonitrile (25 mL, 0.5 L (liter) / mol, with (3- a) as the basis) and water (10mL, 0.2L / mol, based on (3-a)). While stirring with a magnetic stirrer, introduce chlorine gas (2.6mL, liquefied and measured at -70°C, specific gravity 1.64 (-70°C), 4.3g, 60.5mmol, 120mol%) at 2-5°C for 30 minutes, Stir at the same temperature for 1 hour. As a result of GC analysis (area percentage) of the reaction mixture, the components other than the solvent etc. in the reaction mixture are as follows:
[0303] 3-Chloro-5,5-dimethyl-4,5-dihydroisoxazole (4-a: target product): 98%.
[0304] After the reaction, ethyl acetate (25mL), 1M sodium thiosulfate (Na 2 S 2 o 3 ) aqueous solution (5 mL) and saturated brine (10 mL) and stirred. The organic layer and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com