Vectors for treatment of friedreich's ataxia
A carrier and mutual aid technology, applied in the direction of vectors, the use of vectors to introduce foreign genetic material, gene therapy, etc., can solve the problems that there is no effective treatment for FRDA
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
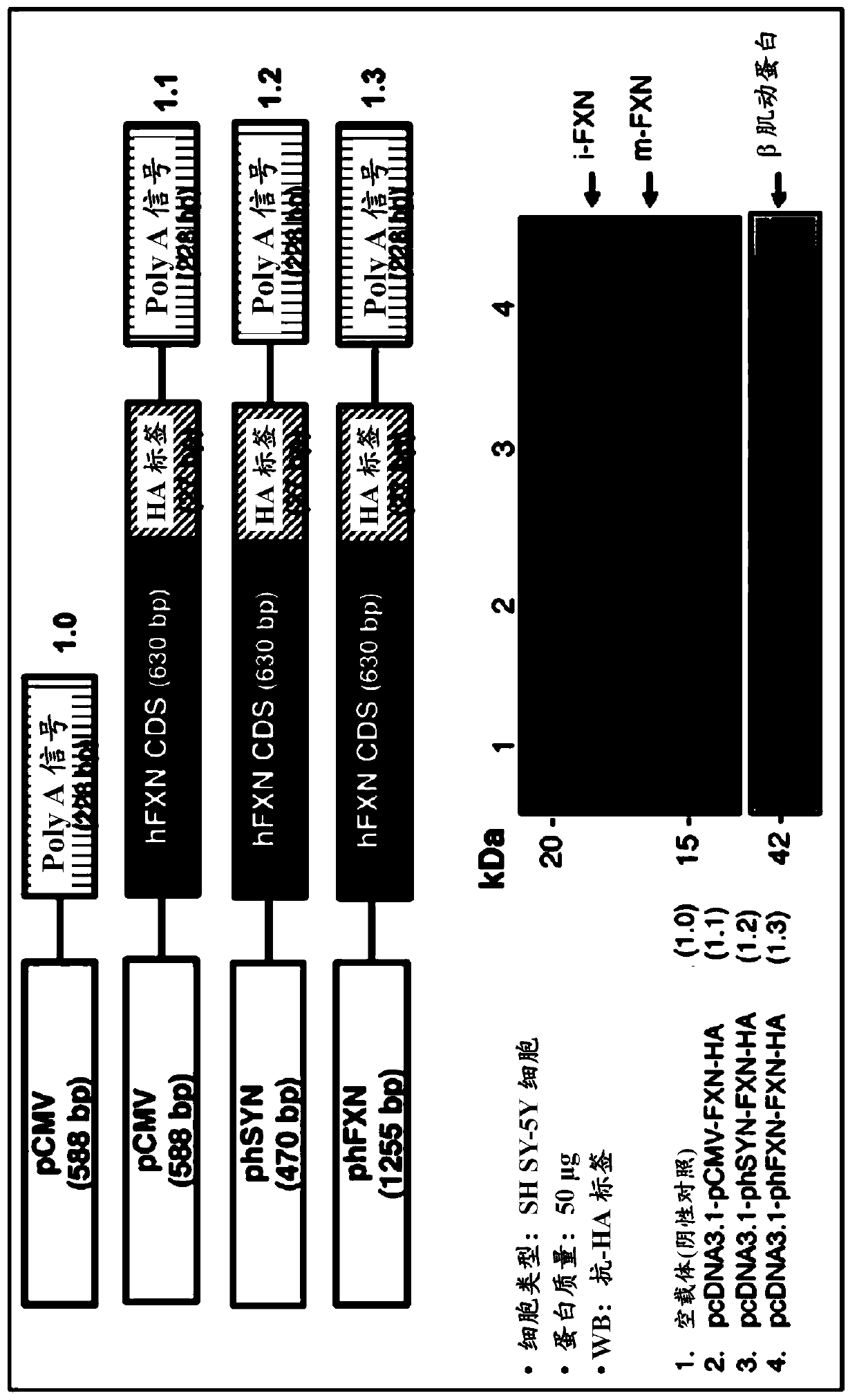
[0097] Example 1: Plasmid construction
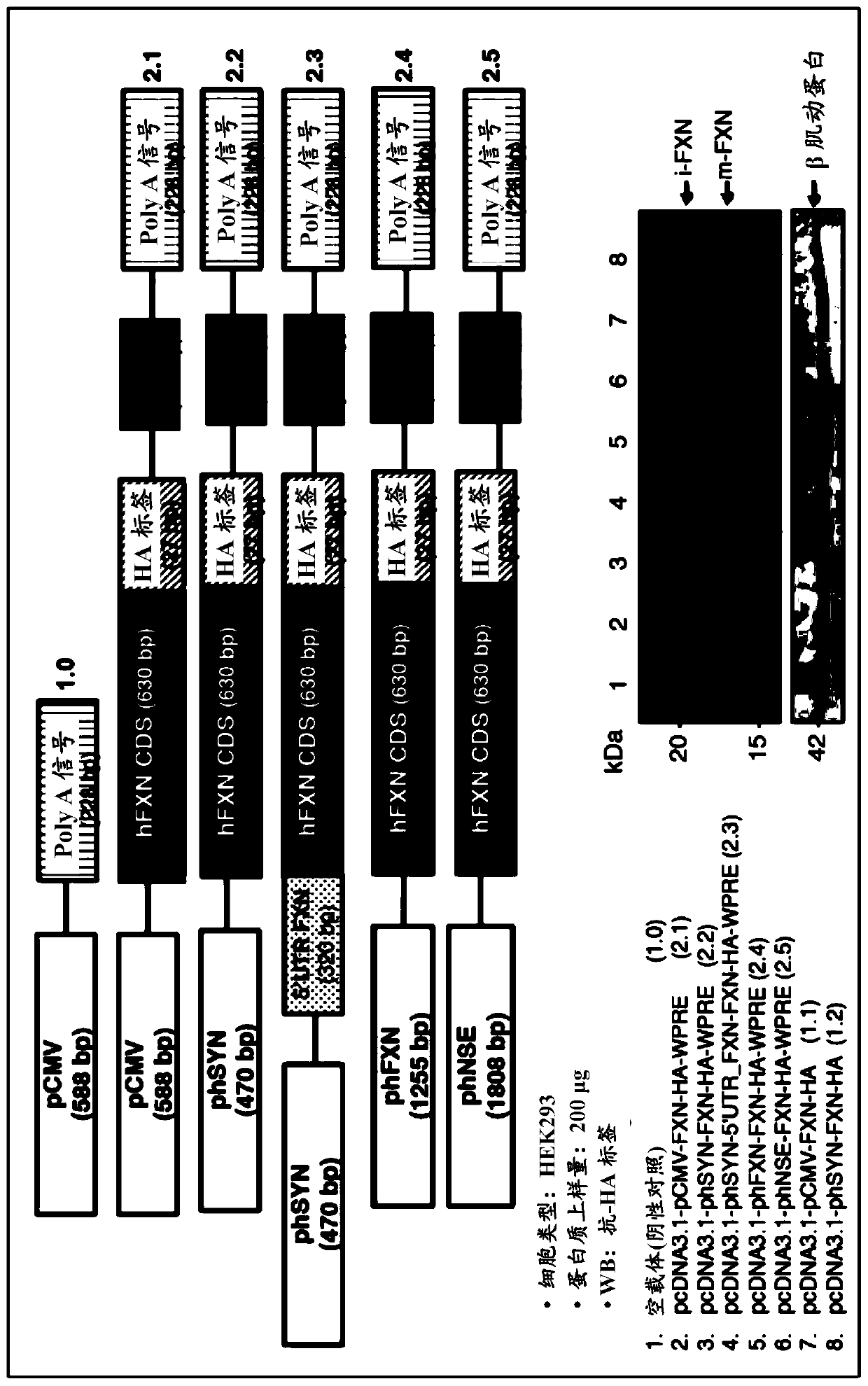
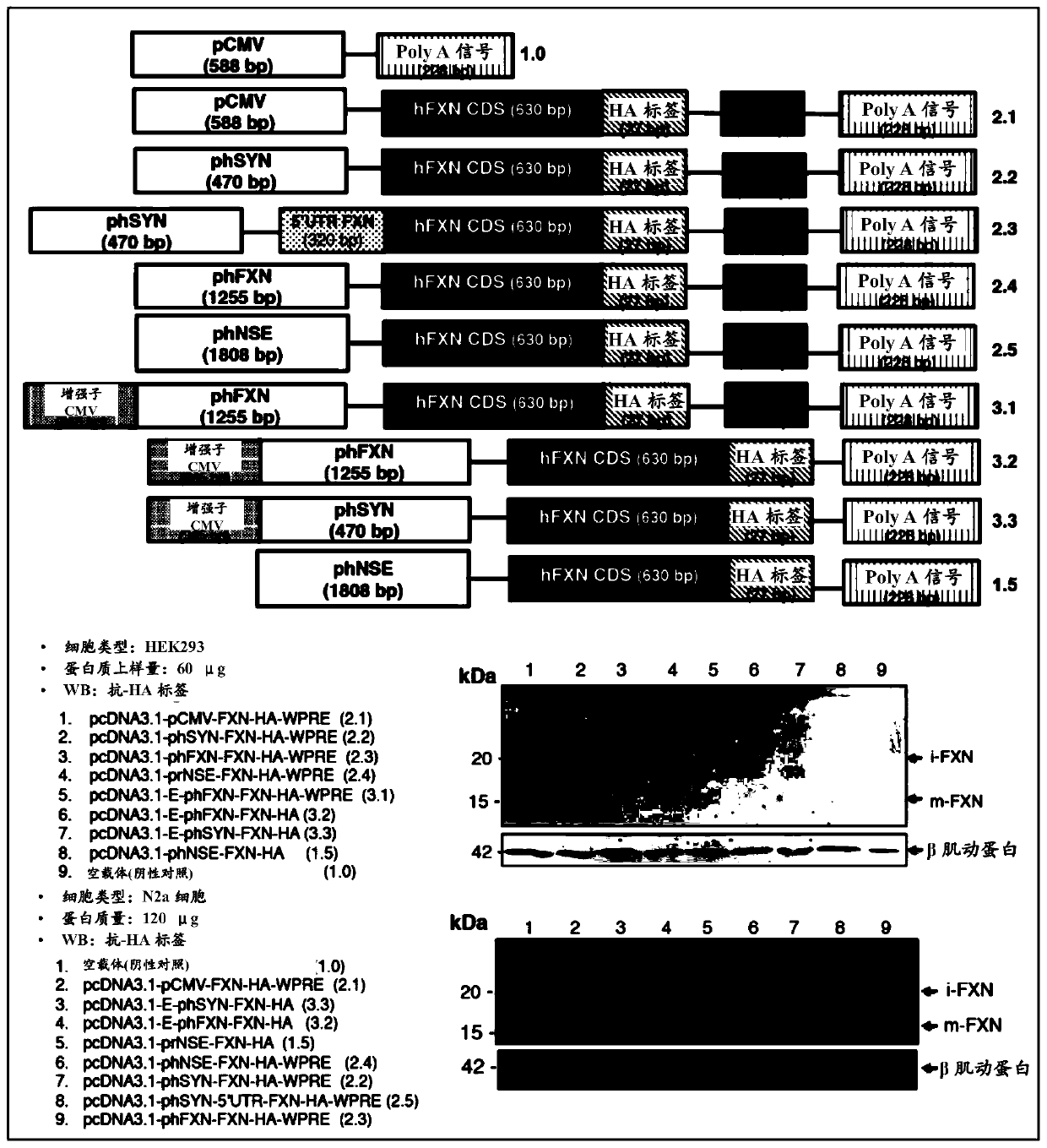
[0098] The coding sequence of isoform 1 of human futaxin (hFXN) was fused to the hemagglutinin tag (HA) and utilized Cloning into the pcDNA3.1 expression vector was performed using the HD cloning kit (Clontech). This fusion system was used for all cloning steps. Several constructs were generated combining CMV or synapsin (phSYN), neuron-specific enolase (phNSE), the 1,255 bp FXN promoter (phFXN1255), or human phosphoglycerate kinase isoform 1 ( The human (h) promoter (p) of phPGK1) was fused to the coding region of the FXN gene. In addition to the woodchuck hepatitis response element (WPRE) sequence at the 3' end, additional regulatory elements such as CMV enhancer and Kozak sequences were added at the 5' end. The constructs generated by all these expression vectors are listed in Table 2.
[0099] Also by replacing the coding sequence of FXN with the firefly luciferase coding sequence (LUC) (amplified from the pGL3-LUC vector (Pr...
Embodiment 2
[0100] Example 2: Construction and preparation of recombinant adeno-associated virus vector.
[0101] The expression cassettes from the pcDNA3.1-phPGK-kFXN-HA-WPRE and pcDNA3.1-phPGK-kLUC-HA-WPRE plasmids were cloned into the SnaBI-MfeI site of the recombinant AAV9 vector (rAAV9-phPGK1-FXN-HA-WPRE vector and rAAV9-phPGK-LUC-WPRE vector). In addition, a control null vector (AAV2 / 9-null) lacking the FXN coding sequence was generated. All three constructs and virus particles were produced by the Vector Production Unit at the Center of Animal Biotechnology and GeneTherapy, Universitat Autònoma de Barcelona. The final titer obtained was 1.4×10 13 vg / ml (AAV9-phPGK-FXN-HA-WPRE), 9.8×10 12 vg / ml (AAV9-phPGK-LUC-WPRE), and 6×10 12 vg / ml (AAV2 / 9-null).
Embodiment 3
[0102] Example 3: Optimizing Musclein Expression
[0103] 1. Cell culture, in vitro expression and luciferase reporter assay
[0104] Dulbecco's Modified Eagle's Medium (DMEM) containing 10% fetal bovine serum (Sigma), 2 mM glutamine, 50 μg / ml penicillin / streptomycin (Life technologies) at a 70% confluency in a 10 cm dish Mouse neuroblastoma cells (N2a), human neuroblastoma cells (SH-SY5Y) and human embryonic kidney (HEK 293) cells were cultured in medium. Transfection was performed using lipofectamine 2000 (Life technologies) for N2a and SH-SY5Y cells and calcium phosphate for HEK 293, using only 4 μg of plasmid DNA in addition to 0.25 μg of EGFP. After transfection, the medium was replaced with fresh DMEM medium (for HEK cells) and Neurobasal, B27 supplement, 10 μM retinoic acid, 2 mM glutamine, 50 μg / ml penicillin / streptomycin (for N2a and SH- SY5Y cells). Forty-eight hours after changing the cell culture medium, cells transfected with the expression plasmids listed in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com