Modulators of irf4 expression
A nucleobase and oligonucleotide technology, applied in DNA/RNA fragments, medical preparations containing active ingredients, peptide/protein components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
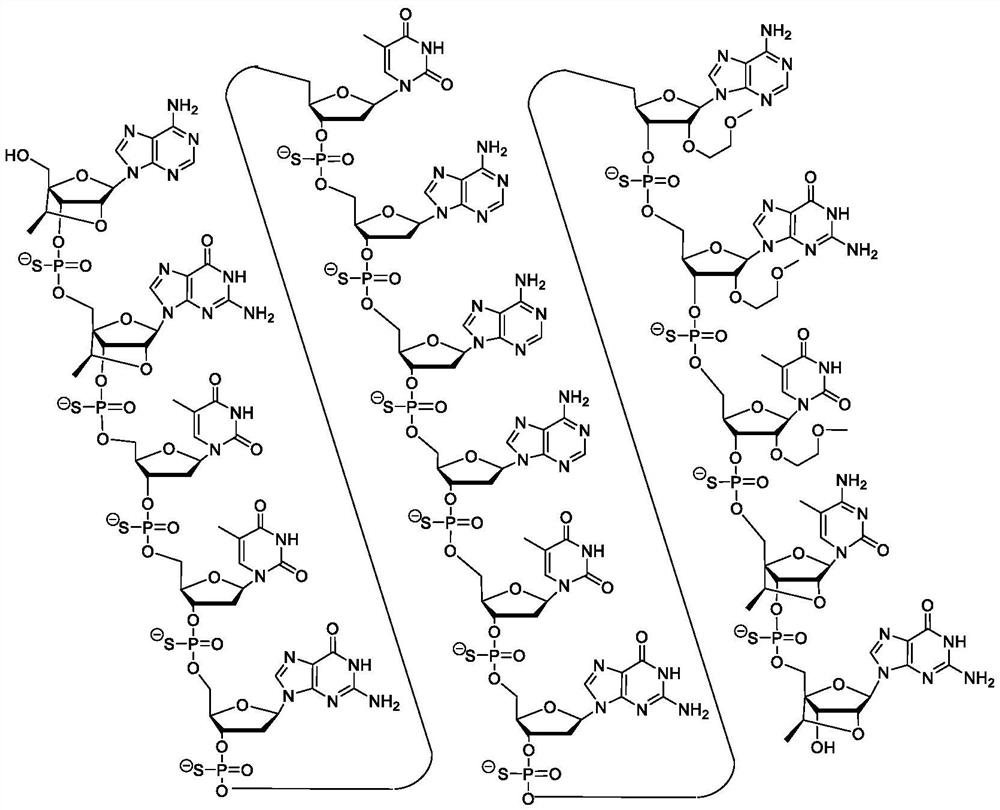
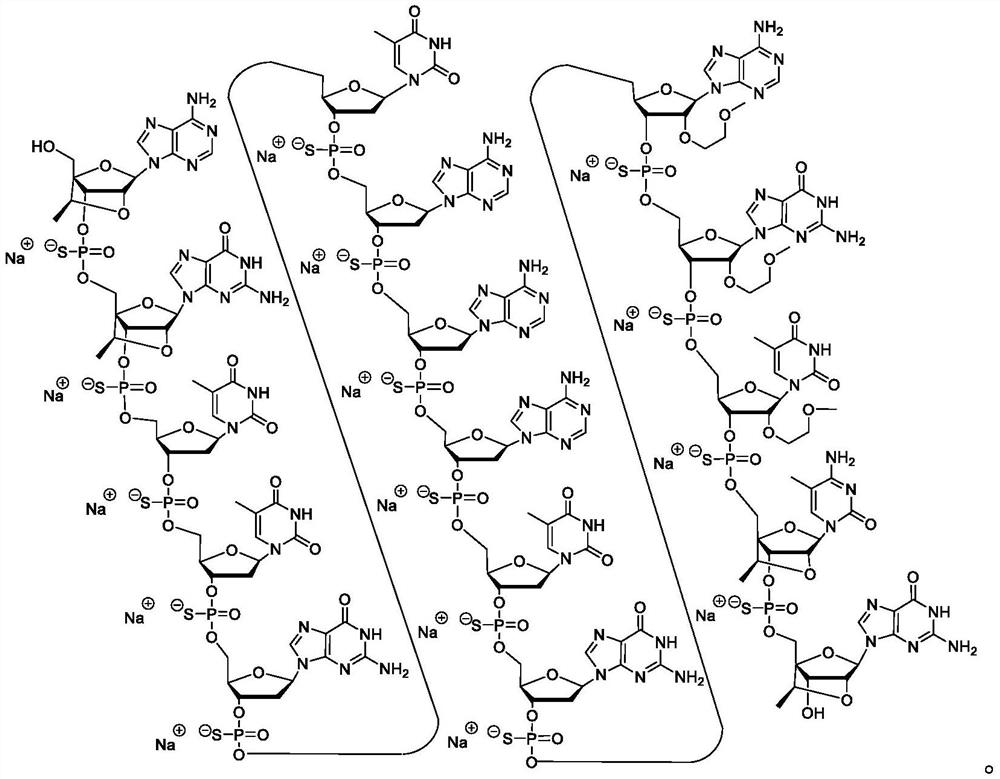
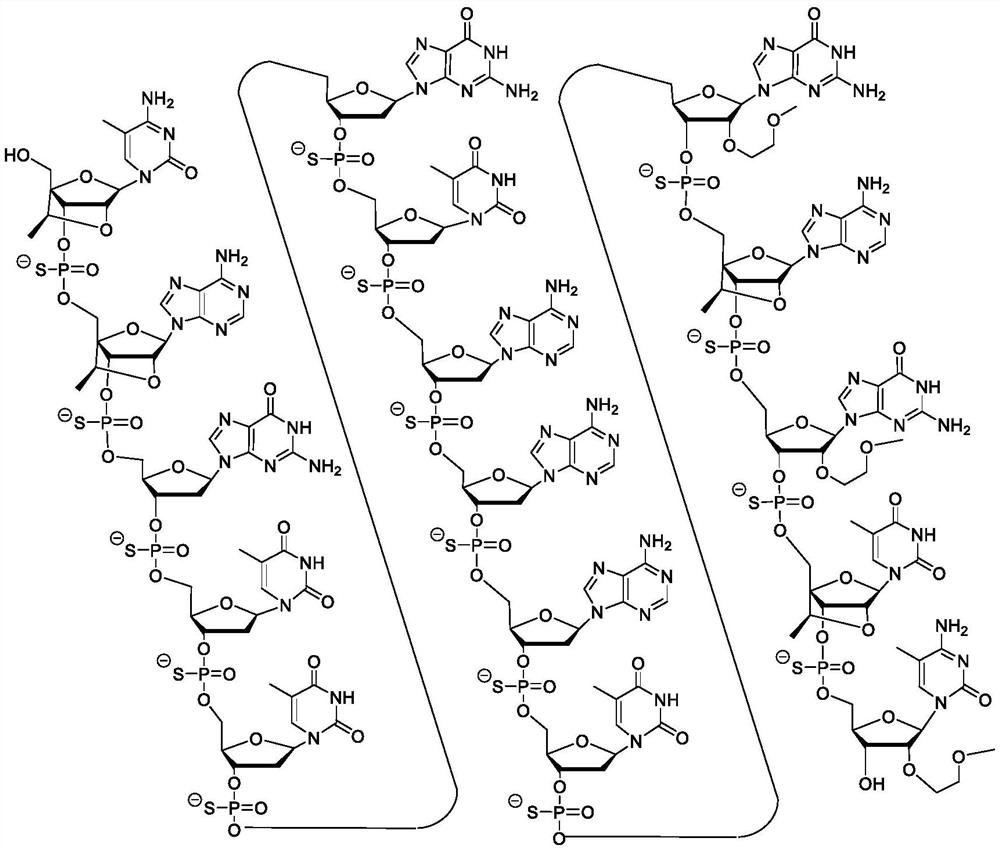
[0112] Certain embodiments provide methods, compounds, and compositions for inhibiting IRF4 expression.
[0113] Certain embodiments provide compounds that target IRF4 nucleic acids. In certain embodiments, the IRF4 nucleic acid has the expression set forth in RefSeq or GENBANK Accession Nos. NM_002460.3 or NT_034880.3_TRUNC_328000_354000 (herein incorporated by reference, disclosed herein as SEQ ID NO: 1 and SEQ ID NO: 2, respectively). sequence. In certain embodiments, the compound is an antisense compound or an oligomeric compound. In certain embodiments, the compounds are single chain compounds. In certain embodiments, the compounds are double-stranded compounds.
[0114] Certain embodiments provide a kind of compound, it comprises the oligonucleotide of modification, the length of described oligonucleotide of modification is the nucleoside of 8 to 80 connections and has and comprises nucleobase sequence SEQ ID NO: A nucleobase sequence of at least 8 contiguous nucleob...
Embodiment 1
[0435] Example 1: Effect of 5-10-5 MOE spacer with phosphorothioate internucleoside linkage on human IRF4 in vitro, single dose
[0436] Modified oligonucleotides complementary to human IRF4 nucleic acid were designed and tested for their effect on IRF4 mRNA in vitro.
[0437] Using electroporation, cultured SK-MEL-28 cells were transfected at a density of 60,000 cells per well with the modified oligonucleotide at a concentration of 20,000 nM or no modified oligonucleotide for the untreated control. After approximately 24 hours, RNA was isolated from the cells and IRF4 mRNA levels were measured by quantitative real-time PCR. Human primer probe set RTS3114 (forward sequence AAGCCTTGGCGTTCTCAGACT, designated herein as SEQ ID NO: 3386; reverse sequence TCAGCTCCTTCACGAGGATTTC, herein designated as SEQ ID NO: 3387; probe sequence CCGGCTGCACATCTGCCTGTACTACC, herein designated as SEQ ID NO: 3387; ID NO:3388) was used to measure mRNA levels. According to as passed Measured total R...
Embodiment 2
[0444] Example 2: Effect of 3-10-3cEt spacer with phosphorothioate internucleoside linkage on human IRF4 in vitro, single dose
[0445] Modified oligonucleotides complementary to human IRF4 nucleic acid were designed and tested for their effect on IRF4 mRNA in vitro.
[0446] Using electroporation, cultured SK-MEL-28 cells were transfected at a density of 20,000 cells per well with the modified oligonucleotide at a concentration of 4,000 nM or no modified oligonucleotide for untreated controls. After approximately 24 hours, RNA was isolated from the cells and IRF4 mRNA levels were measured by quantitative real-time PCR. The human primer probe set RTS3114 (described herein above in Example 1) was used to measure mRNA levels. According to as passed Measured total RNA content regulates IRF4 mRNA levels. Results are shown in the table below as percent control relative to the amount of IRF4 mRNA of untreated control (UTC) cells.
[0447] The modified oligonucleotides in Tables...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com