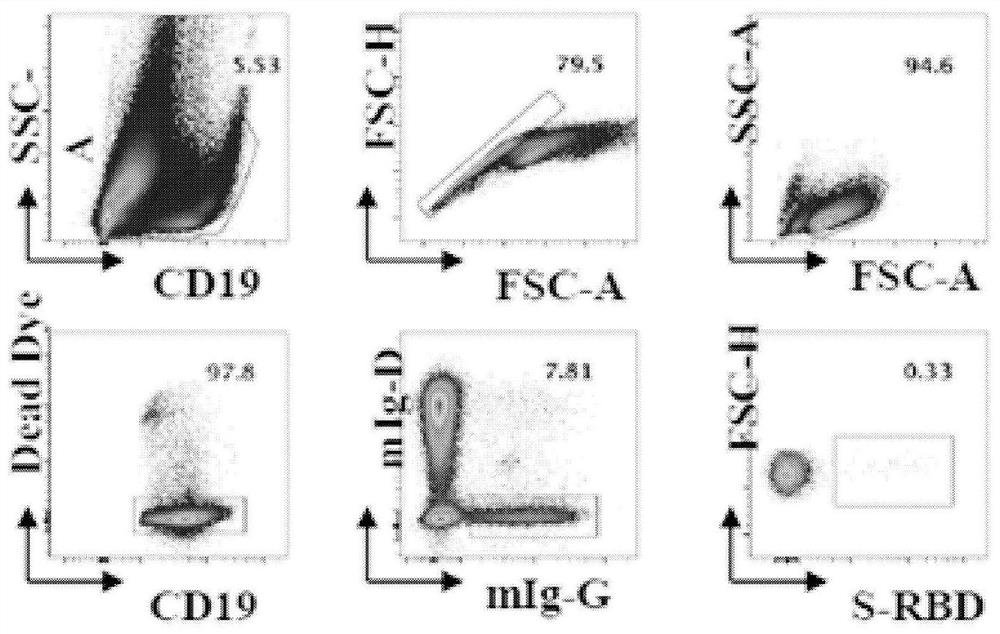
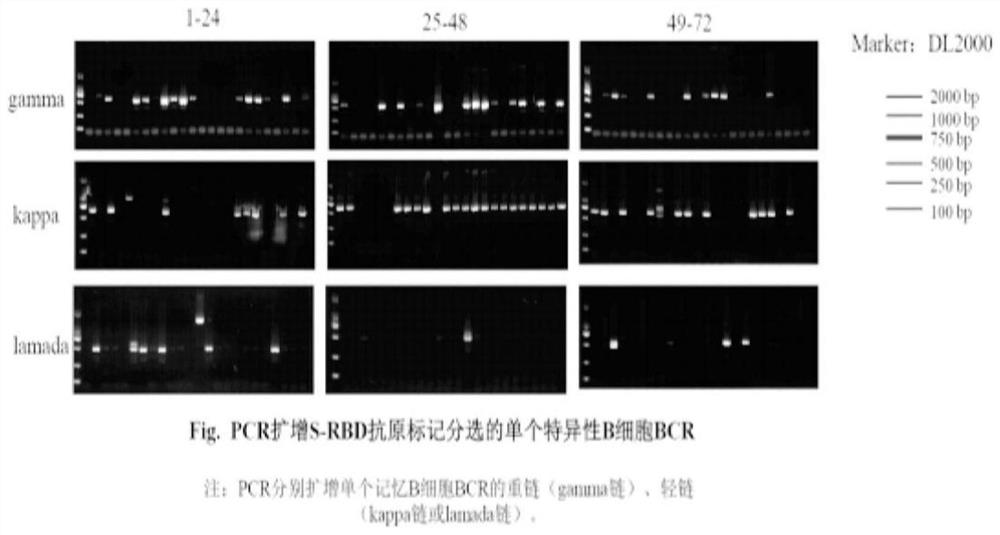
New coronavirus RBD specificity monoclonal antibody and application
A monoclonal antibody and specific technology, applied in the direction of antibodies, applications, antiviral agents, etc., can solve the problem of limited virus blocking effect, achieve the effect of improving specific binding ability and wide application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] This embodiment provides a novel coronavirus RBD-specific monoclonal antibody, the amino acid sequence of the heavy chain is shown in SEQ ID NO:1; the amino acid sequence of the light chain is shown in SEQ ID NO:2.
[0022] This embodiment also provides the application of the above-mentioned novel coronavirus RBD-specific monoclonal antibody in the preparation of reagents or drugs for detecting or diagnosing SARS-CoV-2.
[0023] In actual production, the RBD-specific monoclonal antibody obtained in this embodiment can be used to prepare nucleic acid molecules, or to prepare expression cassettes, recombinant vectors, recombinant bacteria, or transgenic cell lines containing the nucleic acid molecules, or to prepare pharmaceutical compositions. The drug includes the above-mentioned novel coronavirus RBD-specific monoclonal antibody and pharmaceutically acceptable excipients, diluents or carriers.
[0024] In application, this embodiment obtains related products prepared b...
Embodiment 2-20
[0026] The difference between Example 2-20 and Example 1 is that the amino acid sequence of the RBD-specific monoclonal antibody is different, and the amino acid sequence of Example 2-20 is shown in the following table:
[0027] test group heavy chain amino acid sequence light chain amino acid sequence Example 1 - CQTS001 SEQ ID NO:1 SEQ ID NO:2 Embodiment 2-CQTS002 SEQ ID NO:3 SEQ ID NO:4 Embodiment 3-CQTS003 SEQ ID NO:5 SEQ ID NO:6 Embodiment 4-CQTS004 SEQ ID NO:7 SEQ ID NO:8 Embodiment 5-CQTS005 SEQ ID NO:9 SEQ ID NO:10 Embodiment 6-CQTS006 SEQ ID NO: 11 SEQ ID NO:12 Embodiment 7-CQTS007 SEQ ID NO: 13 SEQ ID NO: 14 Embodiment 8-CQTS008 SEQ ID NO: 15 SEQ ID NO: 16 Embodiment 9-CQTS009 SEQ ID NO: 17 SEQ ID NO: 18 Embodiment 10-CQTS010 SEQ ID NO: 19 SEQ ID NO: 20 Embodiment 11-CQTS011 SEQ ID NO: 21 SEQ ID NO: 22 Example 12 - CQTS012 SEQ ID NO: 23 SEQ ID NO: 24...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com