Fibrin glue composite system loaded with cisplatin
A technology of fibrin glue and fibrinogen, applied in the direction of drug combination, non-active ingredients of polymer compounds, medical preparations containing active ingredients, etc., can solve the problems of non-targeted distribution, potential safety hazards, toxic and side effects, etc. To achieve the effect of inhibiting proliferation, inhibiting recurrence, and less toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: Preparation of fibrin glue (Fibrin gel)
[0039] 1. Preparation of thrombin solution
[0040] Thrombin was purchased from Dalian Meilun Biotechnology Co., Ltd., product number MB1368, molecular weight 37kD, CASNo.9002-0404.
[0041] Add thrombin to 40mmol / L calcium chloride aqueous solution to dissolve, prepare a thrombin solution with a concentration of 100 units / mL, and store at -20°C.
[0042] 2. Preparation of fibrinogen solution
[0043] Fibrinogen was purchased from Dalian Meilun Biotechnology Co., Ltd., product number MB5809, molecular weight 340kDa, CAS No.9001-32-5.
[0044] The fibrinogen was dissolved in 0.9% physiological saline to prepare a fibrinogen solution with a concentration of 10 mg / mL, and stored at -20°C.
[0045] 3. Preparation of fibrin glue
[0046] The above fibrinogen solution and thrombin solution were mixed according to the volume ratio of 9:1 to form fibrin glue.
Embodiment 2
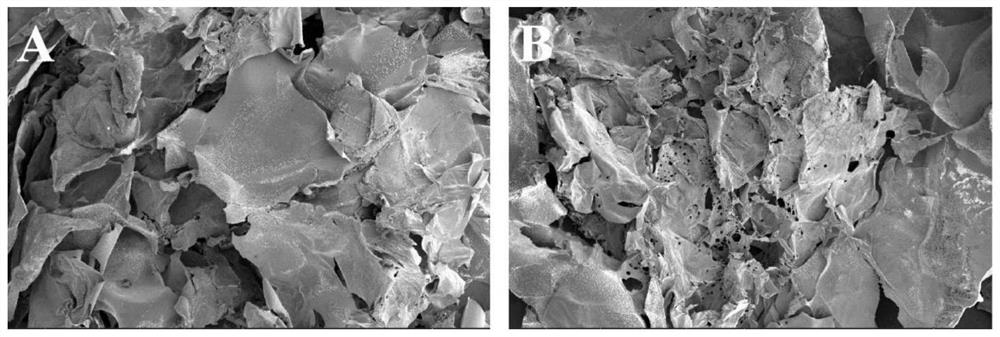
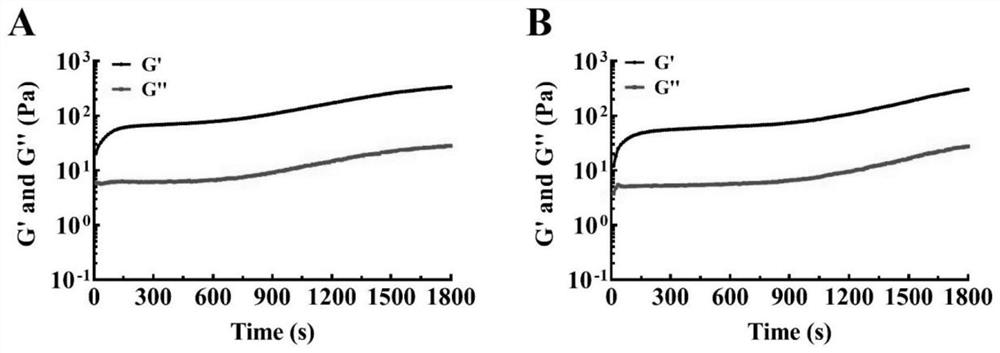
[0047] Embodiment 2: Preparation of cisplatin / fibrin glue (CDDP / Fibrin gel) composite system
[0048] 1. Preparation of thrombin solution
[0049] Using the same method as in Step 1 of Example 1, a thrombin solution with a concentration of 100 units / mL was prepared and stored at -20°C.
[0050] 2. Preparation of fibrinogen solution
[0051] Using the same method as in Step 2 of Example 1, a fibrinogen solution with a concentration of 10 mg / mL was prepared and stored at -20°C.
[0052] 3. Configure CDDP solution
[0053] Cisplatin (CDDP) was purchased from Dalian Meilun Biotechnology Co., Ltd.
[0054] Add CDDP to 0.9% physiological saline to dissolve, prepare a CDDP solution with a concentration of 2 mg / mL, and store at -20°C.
[0055] 4. Preparation of CDDP / Fibringel composite system
[0056] The above-mentioned fibrinogen solution, thrombin solution and CDDP solution were mixed at a volume ratio of 9:1:1 to obtain a CDDP / Fibringel composite system.
Embodiment 3
[0057] Embodiment 3: preparation combined therapy equipment
[0058] The combination therapy equipment of the present invention is composed of two parts: (1) stereotaxic radiotherapy device, (2) drug package. Wherein, the drug package includes the CDDP / Fibringel composite system prepared in Example 2.
[0059] The beneficial effect of the preparation of the present invention is demonstrated by the following experimental examples.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com