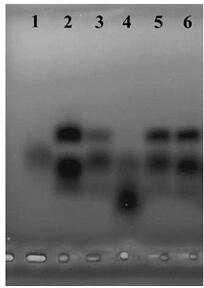
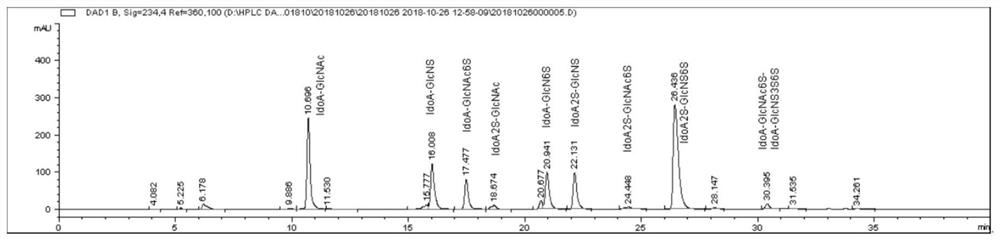
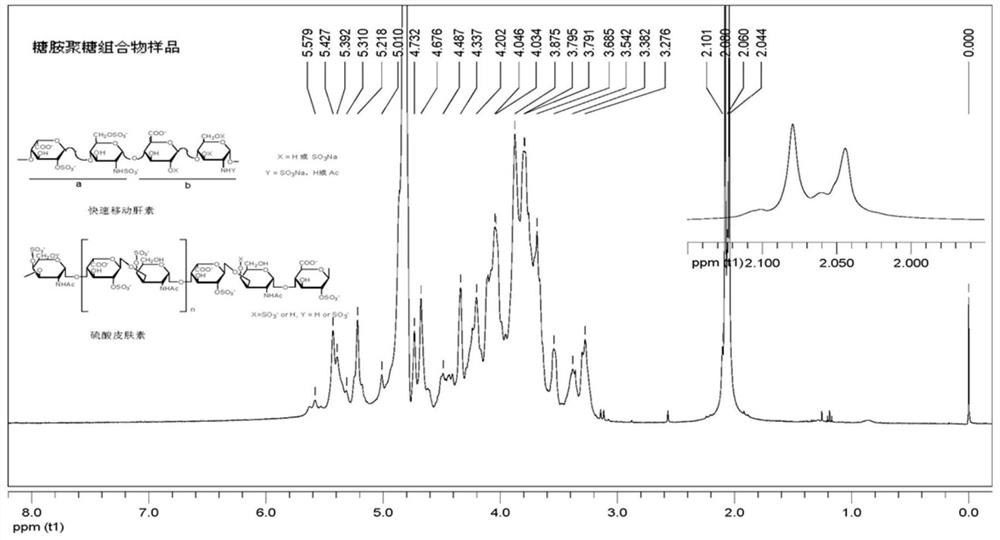
Glycosaminoglycan composition and preparation method and application thereof
A glycosaminoglycan and composition technology, applied in the field of glycosaminoglycan composition and its preparation, can solve problems such as easy risk, complicated supply chain, affecting the quality or supply of heparin products, and achieve prevention and repair of vascular lesions , significant economic effect, and low bleeding risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Preparation of Glycosaminoglycan Composition 1
[0046] The by-product glycosaminoglycan of sheep intestinal mucosa heparin is obtained in the process of purification and preparation of sheep intestinal mucosa heparin. Specifically include the following steps, crude sheep heparin sodium (manufacturer: Shandong Shenlian Biotechnology Co., Ltd., batch number: 20150326, obtained from sheep intestinal mucosa extraction), heparin purification adopts a well-known process in the industry, that is, dissolve the alkali in water and then dissolve the alkali hydrolyzate, and protease hydrolysis, after the hydrolyzate is adjusted to a weak base, it is adsorbed and eluted with anion exchange resin, and alcohol precipitation is used to prepare sheep heparin, and the by-product glycosaminoglycan of sheep heparin is obtained after separation of heparin. Add ethanol to the supernatant to a final mass concentration of 70%, recover the precipitate, and dry it.
[0047] Weigh 8...
Embodiment 2
[0048] Example 2: Preparation of Glycosaminoglycan Composition 2
[0049] Take the by-product glycosaminoglycan in the purification of bovine intestinal mucosal heparin, which is obtained when the crude bovine intestinal mucosal heparin is refined and purified (crude bovine intestinal mucosal heparin is extracted from the small intestine mucous membranes), obtained from the glycosaminoglycan-rich supernatant during the heparin process, obtained by precipitation with a final concentration of 70% ethanol and dried.
[0050] Weigh 100 g of bovine intestinal mucosal heparin by-product glycosaminoglycan, stir and dissolve with 1000 mL of 2% saline, then add 40 g of sodium hydroxide, and react at 25°C for 2 hours. Adjust the pH to 7.0 with dilute hydrochloric acid, add 1 g of potassium permanganate, and stir for 30 minutes. Then add 100 g of diatomaceous earth, stir for 5 min and then filter with filter paper. Add 2.2g ETDA to the filtrate, and react at room temperature for 4h. C...
Embodiment 3
[0051] Example 3: Preparation of Glycosaminoglycan Composition 3
[0052] Take 30 g of glycosaminoglycan, a by-product of bovine lung heparin, and dissolve it with 500 mL of 3% saline, then add 3 g of sodium hydroxide, and react at 35°C for 2 hours. Adjust the pH to 7.8 with dilute hydrochloric acid, add 10 mL of hydrogen peroxide, stir and let stand for decolorization for 5 hours. Add 30g of diatomaceous earth, stir for 5min and then filter with filter paper. Add 1.3g ETDA to the filtrate, and react at room temperature for 3.5h. Adjust the pH to 5.5, add cold acetone to a final concentration of 41%, stir well and then centrifuge to collect the precipitate. Dissolve the precipitate with 300 mL of 5% sodium chloride solution, adjust the pH to 5.8, add ethanol to a final concentration of 48%, and let stand overnight at room temperature. The supernatant was discarded by centrifugation, the precipitate was dehydrated with 95% ethanol, and dried in vacuum to obtain 14.2 g of the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap