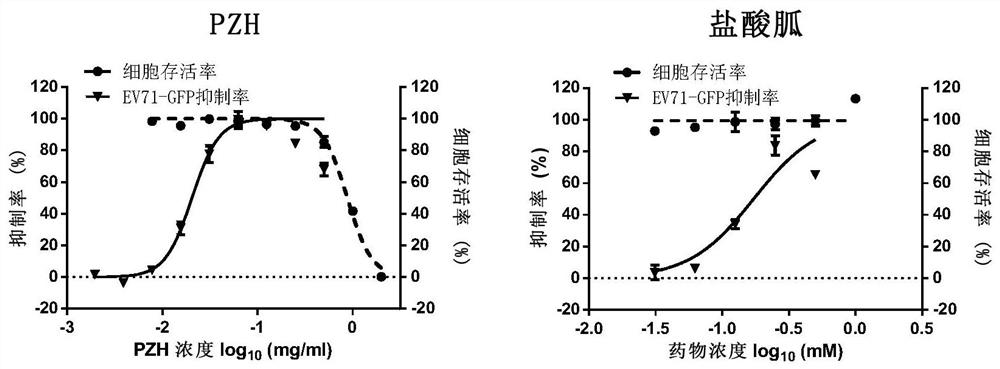
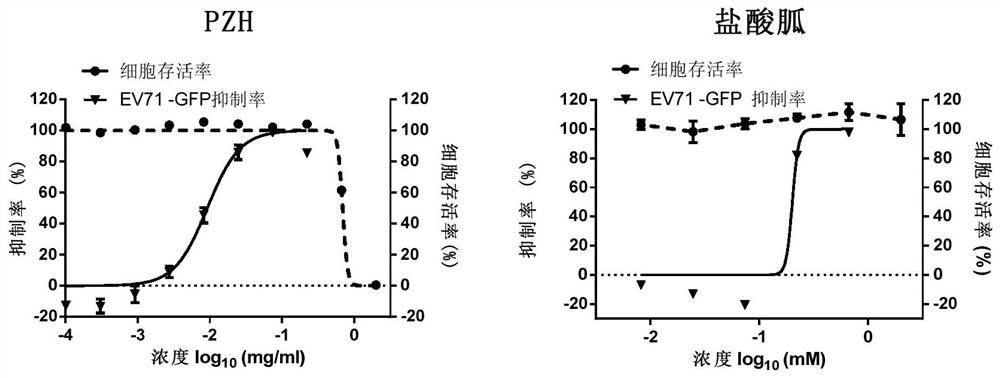
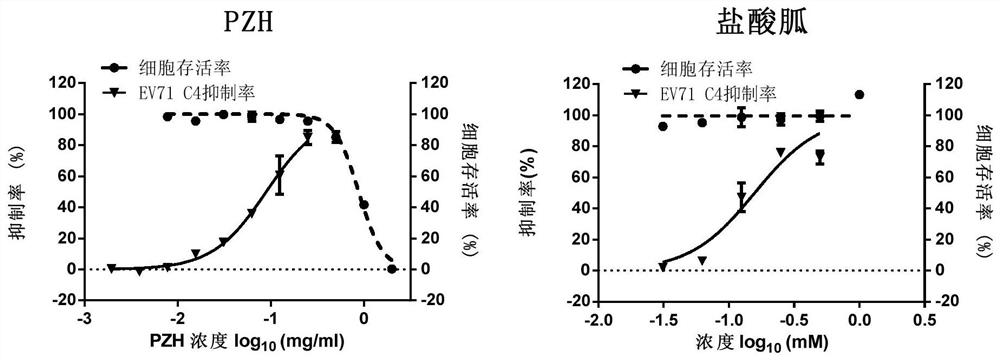
Application of Pien Tze Huang and its preparations in the preparation of drugs for the prevention and treatment of enterovirus ev71 infection
An enterovirus, EV71 technology, applied in antiviral agents, drug combinations, pharmaceutical formulations, etc., can solve the problems of increased drug uncertainty, time-consuming, high risk, etc., and achieve significant anti-enterovirus EV71 infection activity, Effect of increasing cell viability and reducing production level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Pien Tze Huang (lozenge, 3g / capsule) is used for hand, foot and mouth disease, angina or rash caused by enterovirus EV71 infection, 0.15-0.30 g once for children under 8 years old, 2-3 times a day; 0.3-0.9 g for adults once grams, 2-3 times a day.
Embodiment 2
[0059] Take Pien Tze Huang Capsules (0.3g / capsule, crushed from lozenges into granules and powdered into capsules) for hand, foot and mouth disease, angina or rash caused by enterovirus EV71 infection, 0.15-0.30 g once for children under eight years old , 2-3 times a day; adults 0.3-0.9 g once a day, 2-3 times a day.
Embodiment 3
[0061] Get Pien Tze Huang in Example 1 and pulverize it into granules, add conventional adjuvants and prepare Pien Tze Huang tablets (each containing Pien Tze Huang 0.3g) according to conventional techniques, for hand, foot and mouth disease, angina or rash caused by enterovirus EV71 infection, eight Children under the age of 0.15-0.30 grams once, 2-3 times a day; adults 0.6 grams once, 2-3 times a day.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com