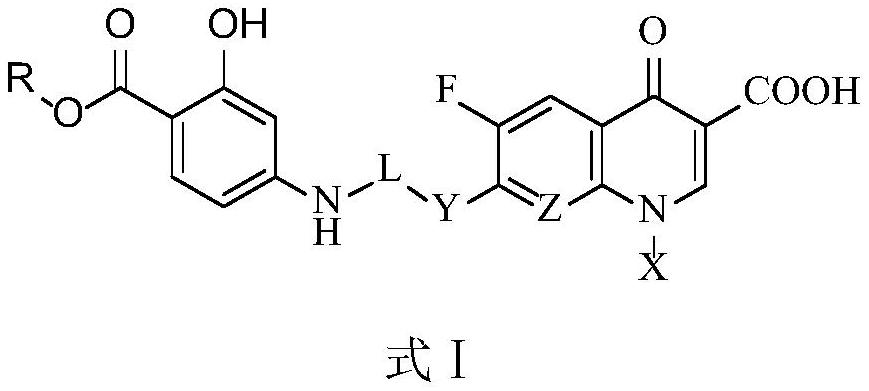
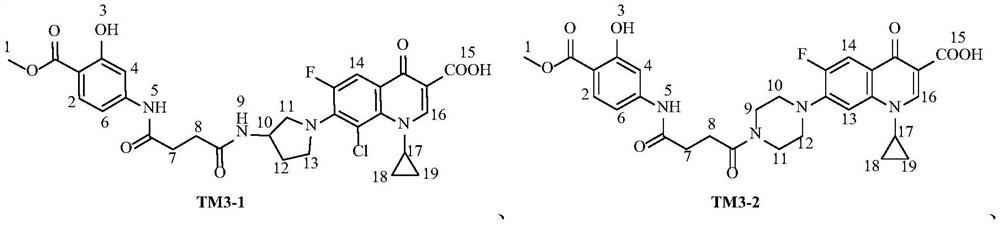
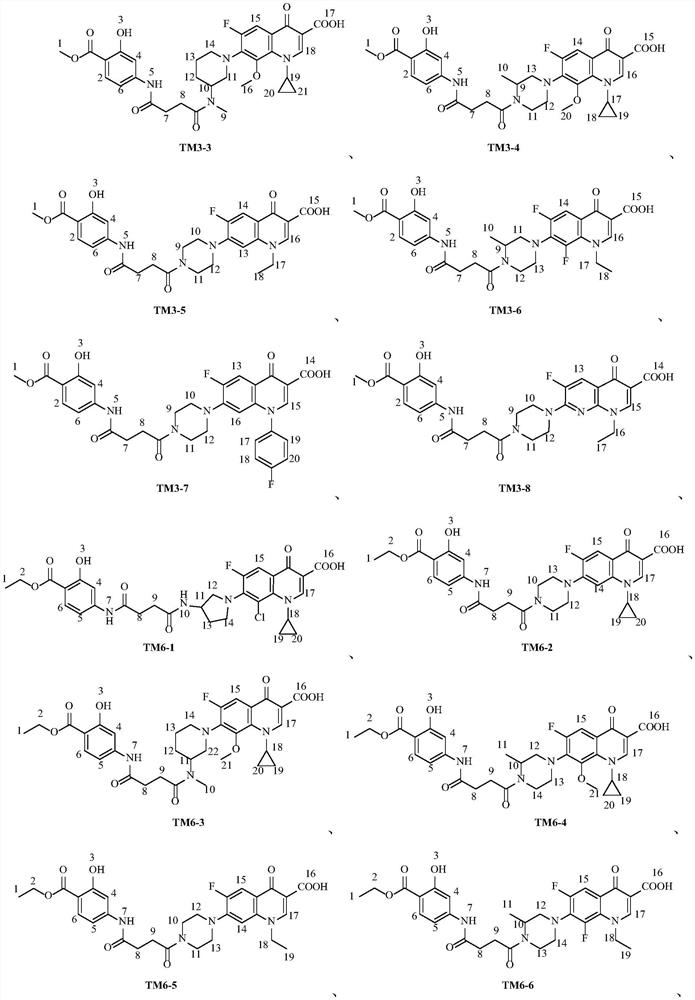
Fluoroquinolone derivatives of p-aminosalicylic acid and their intermediates, preparation methods and applications
A technology of p-aminosalicylic acid and fluoroquinolones, applied in the field of drug synthesis, can solve problems affecting economic benefits, complex differentiation of citrus pathogenic bacteria, and rapid disease spread
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0047] The technical solutions in the embodiments of the present invention will be clearly and completely described below with reference to the embodiments of the present invention. Obviously, the described embodiments are only a part of the embodiments of the present invention, rather than all the embodiments. Based on the embodiments of the present invention, all other embodiments obtained by those of ordinary skill in the art without creative efforts shall fall within the protection scope of the present invention.
[0048] 1. Main reagents and instruments
[0049] Para-aminosalicylic acid, chloroacetyl chloride, dichloromethane, N,N-dimethylformamide, gatifloxacin, clinifloxacin, ciprofloxacin, balofloxacin, sarafloxacin, enoxacin (>95%); Norfloxacin, Lomefloxacin (AR); Concentrated sulfuric acid, methanol, sodium bicarbonate, potassium carbonate, absolute ethanol, succinic anhydride, HBTU (AR), other reagents are commercially available chemicals Pure or analytically pure ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com