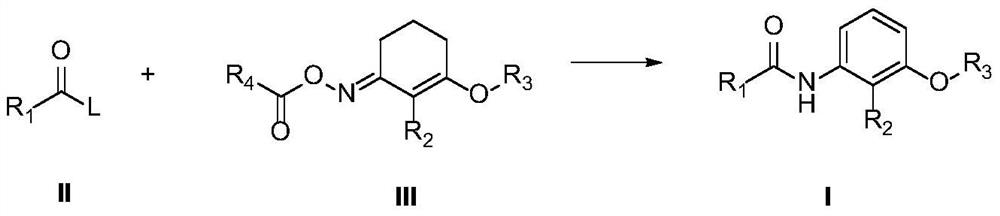
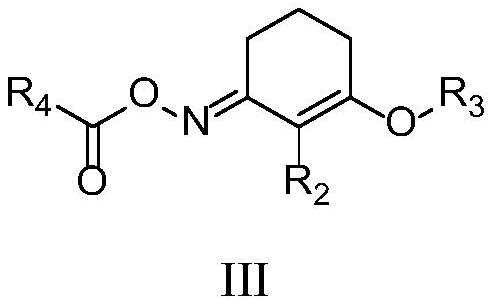
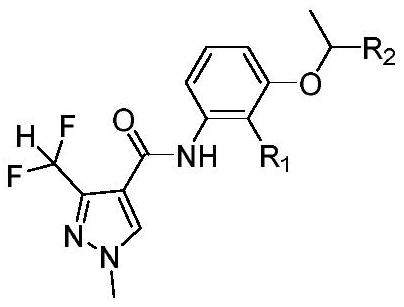
A kind of preparation method of amide compound
A technology for amide compounds and acid halides, which is applied in the field of preparation of amide compounds, can solve the problems of high price of reaction raw materials, many reaction three wastes, difficult treatment, etc., and achieves the effects of high bactericidal activity, reduced pollution, and increased safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Synthesis of 2-methyl-3-(2-pentyloxy)cyclohex-2-en-1-one-O-benzoyl oxime (Compound III-68 in Table 1)
[0054] (1) Synthesis of 2-[(dimethylamino)methylene]-1,3-cyclohexanedione
[0055]
[0056] Add methanol (200.0g), dimethylamine solution (112.8g, 40%) and 1,3-cyclohexanedione (56.0g) into a three-necked flask with a mechanical stirring device, stir at room temperature for 30min, and cool to 0- At 10°C, formaldehyde solution (88.3g, 37%) was slowly added dropwise and stirred for 5h to obtain 2-[(dimethylamino)methylene]-1,3-cyclohexanedione, which was directly used in the next step.
[0057] (2) Synthesis of 2-methyl-1,3-cyclohexanedione
[0058]
[0059] The reaction solution from the previous step was transferred to an autoclave, a palladium carbon catalyst (5.6 g, 10%) was added, and the reaction was carried out at 2 MPa and 30° C. for 8 h. The reaction solution was taken out, the catalyst was filtered off, 200 g of water was added, methanol was distilled ...
Embodiment 2
[0070] Synthesis of 2-methyl-3-(2-pentyloxy)cyclohex-2-en-1-one-O-pivaloyl oxime (Compound III-67 in Table 1)
[0071]
[0072] Add intermediate 2-methyl-3-(2-pentyloxy)cyclohex-2-en-1-one oxime (21.1g), triethylamine (10.1g), toluene (200mL) to the reaction flask, Pivaloyl chloride (12.1 g) was added dropwise thereto, and stirred at room temperature for 3 h after dropping. The reaction solution was cooled to room temperature, and water (100 mL) was added, and stirred at this temperature for 30 min, and then the layers were separated. The oil layer was precipitated to obtain 28.4 g of intermediate 2-methyl-3-(2-pentyloxy)cyclohex-2-en-1-one-O-pivaloyl oxime, yield: 96% (as 2-methyl Base-3-(2-pentyloxy)cyclohex-2-en-1-one oxime calculation).
Embodiment 3
[0074] Synthesis of 2-methyl-3-(2-pentyloxy)cyclohex-2-en-1-one-O-acetyloxime (Compound III-65 in Table 1)
[0075]
[0076]Add intermediate 2-methyl-3-(2-pentyloxy)cyclohex-2-en-1-one oxime (21.1g), triethylamine (10.1g), toluene (200mL) to the reaction flask, Acetyl chloride (7.9 g) was added dropwise thereto, and stirred at room temperature for 3 h after the drop was completed. The reaction solution was cooled to room temperature, and water (100 mL) was added, and stirred at this temperature for 30 min, and then the layers were separated. The oil layer was precipitated to obtain 24.8 g of the intermediate 2-methyl-3-(2-pentyloxy)cyclohex-2-en-1-one-O-acetyl oxime, yield: 98% (as 2-methyl- 3-(2-pentyloxy)cyclohex-2-en-1-one oxime).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com