Fucose-binding protein, method for producing same, and use of same
A technology of binding and fucose, applied in the field of fucose-binding proteins, can solve problems such as non-existence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
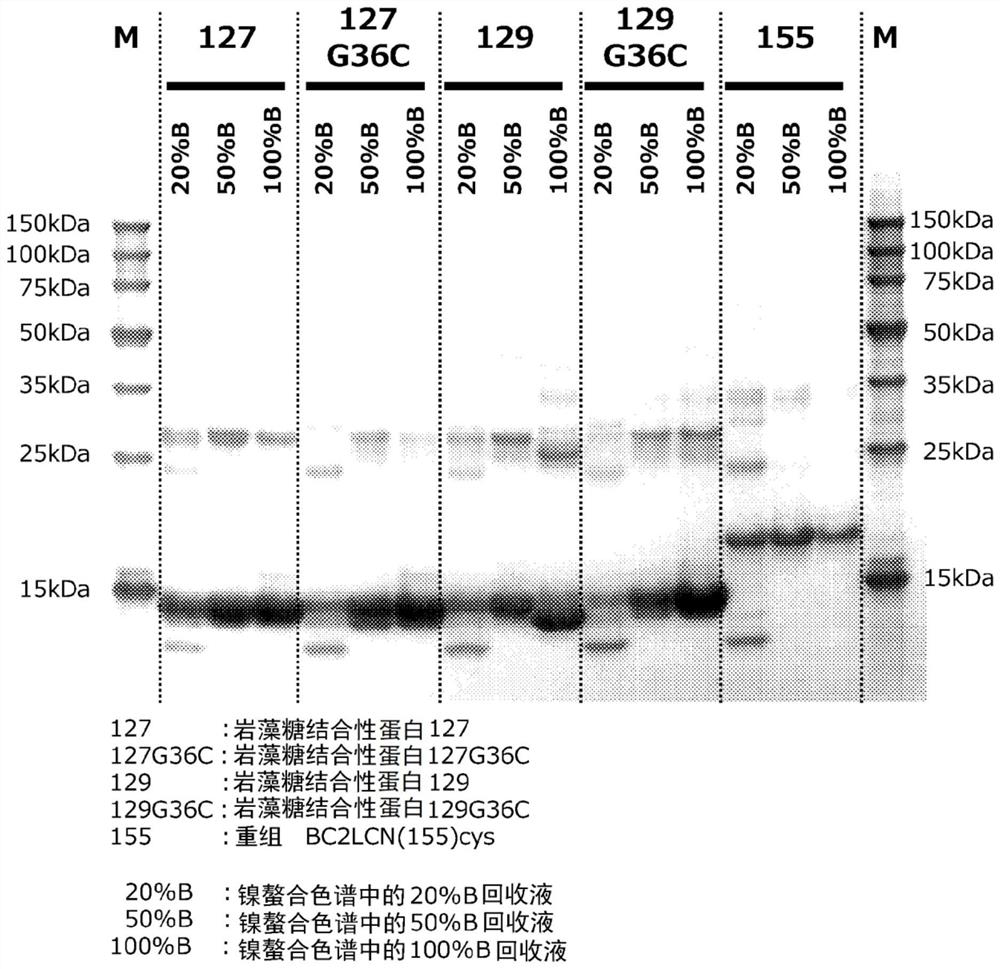
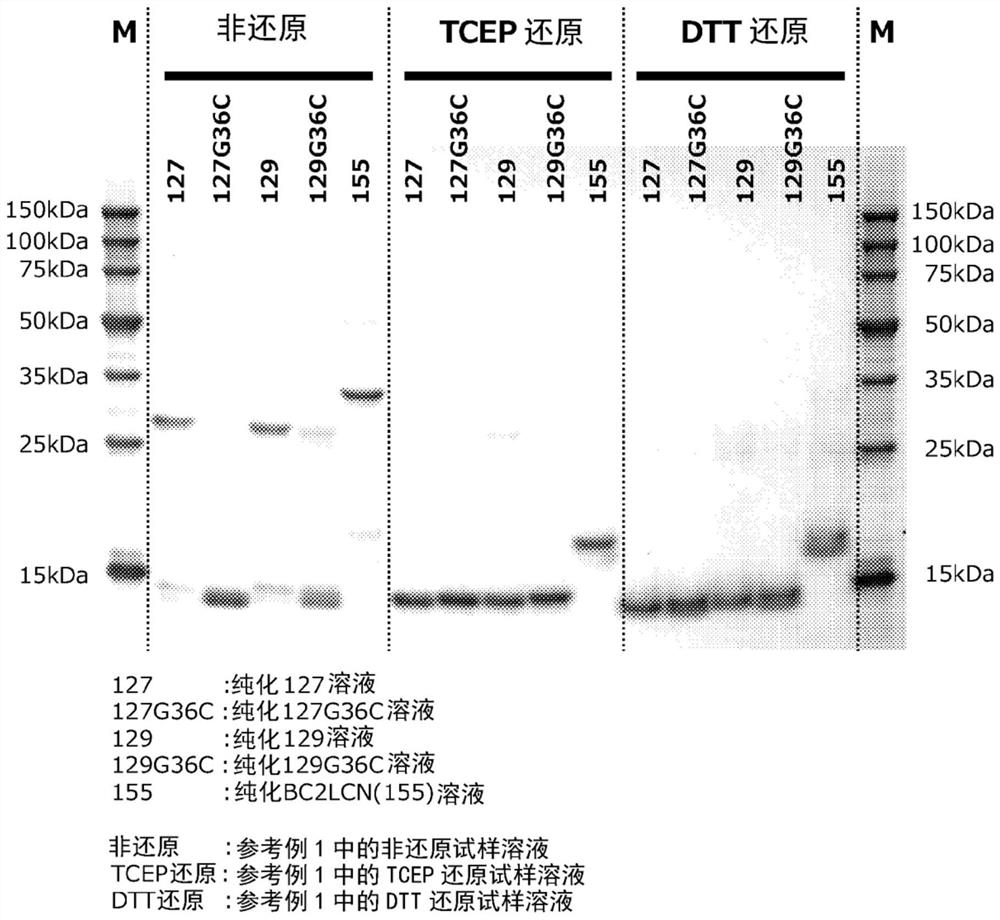
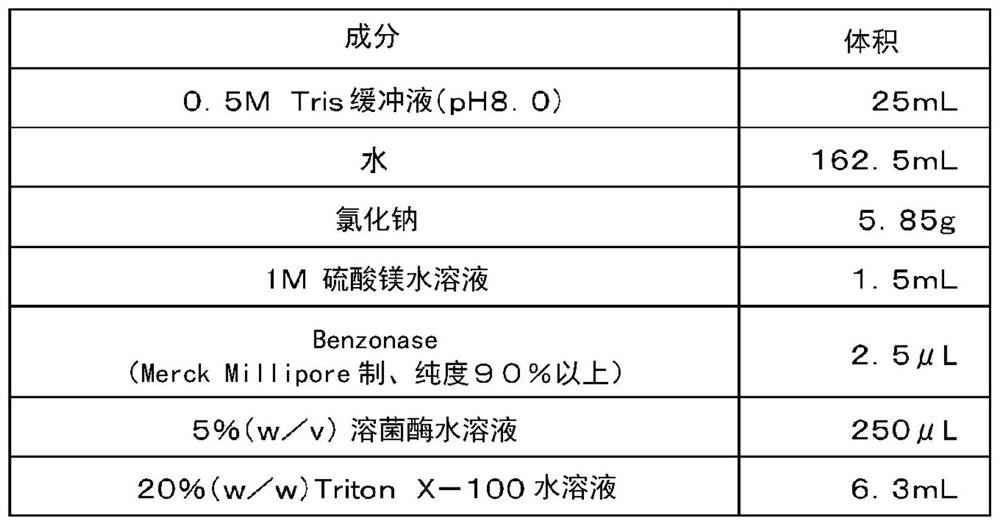
[0226] Example 1 Production of fucose-binding protein 129 and evaluation of productivity
[0227] Example 1 involves adding an oligopeptide containing a polyhistidine sequence to the N-terminus of the amino acid sequence of the fucose-binding protein shown in SEQ ID NO: 2 consisting of 129 amino acid residues, and adding an oligopeptide containing cysteine to the C-terminus. Production and productivity evaluation of fucose-binding protein (hereinafter referred to as fucose-binding protein 129) of oligopeptide residues.
[0228] (1) Production of expression vector pET-BC2LCN(129)cys and recombinant Escherichia coli BL21(DE3) / pET-BC2LCN(129)cys
[0229] The expression vector pET-BC2LCN(129)cys is an expression vector for expressing fucose-binding protein 129. The amino acid sequence of fucose-binding protein 129 is SEQ ID NO: 33, the 5th to 10th positions are polyhistidine sequences, the 15th to 143rd positions are the amino acid sequence of SEQ ID NO: 2, and the 144th to 14t...
Embodiment 2
[0236] Example 2 Production of fucose-binding protein 127 and evaluation of productivity
[0237] Example 2 involves adding an oligopeptide containing a polyhistidine sequence to the N-terminus of the amino acid sequence of the fucose-binding protein shown in SEQ ID NO: 3 consisting of 127 amino acid residues, and adding an oligopeptide containing cysteine to the C-terminus Production and productivity evaluation of fucose-binding protein (hereinafter referred to as fucose-binding protein 127) of oligopeptide residues.
[0238] (1) Production of expression vector pET-BC2LCN(127)cys and recombinant Escherichia coli BL21(DE3) / pET-BC2LCN(127)cys
[0239] The expression vector pET-BC2LCN(127)cys is an expression vector for expressing fucose-binding protein 127. The amino acid sequence of fucose-binding protein 127 is SEQ ID NO: 34, the 5th to 10th positions are polyhistidine sequences, the 15th to 141st positions are the amino acid sequence of SEQ ID NO: 3, and the 142nd to 14th...
Embodiment 3
[0246] Example 3 Production of fucose-binding protein 129G36C and evaluation of productivity
[0247] Example 3 relates to the amino acid sequence of the fucose-binding protein shown in SEQ ID NO: 4 consisting of 129 amino acid residues (where the glycine residue at position 36 in SEQ ID NO: 2 is replaced with a cysteine residue Amino acid sequence) to which an oligopeptide containing a polyhistidine sequence is added to the N-terminus, and an oligopeptide containing a cysteine residue is added to the C-terminus of a fucose-binding protein (hereinafter referred to as fucose-binding protein 129G36C.) Evaluation of manufacturing and productivity.
[0248] (1) Production of expression vector pET-BC2LCN(129G36C)cys and recombinant Escherichia coli BL21(DE3) / pET-BC2LCN(129G36C)cys
[0249] The expression vector pET-BC2LCN(129G36C)cys is an expression vector for expressing fucose-binding protein 129G36C. The amino acid sequence of the fucose-binding protein 129G36C is SEQ ID N...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com