Compositions and methods for treating non-alcoholic steatohepatitis
A non-alcoholic, fatty liver technology, applied in drug combinations, active ingredients of heterocyclic compounds, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
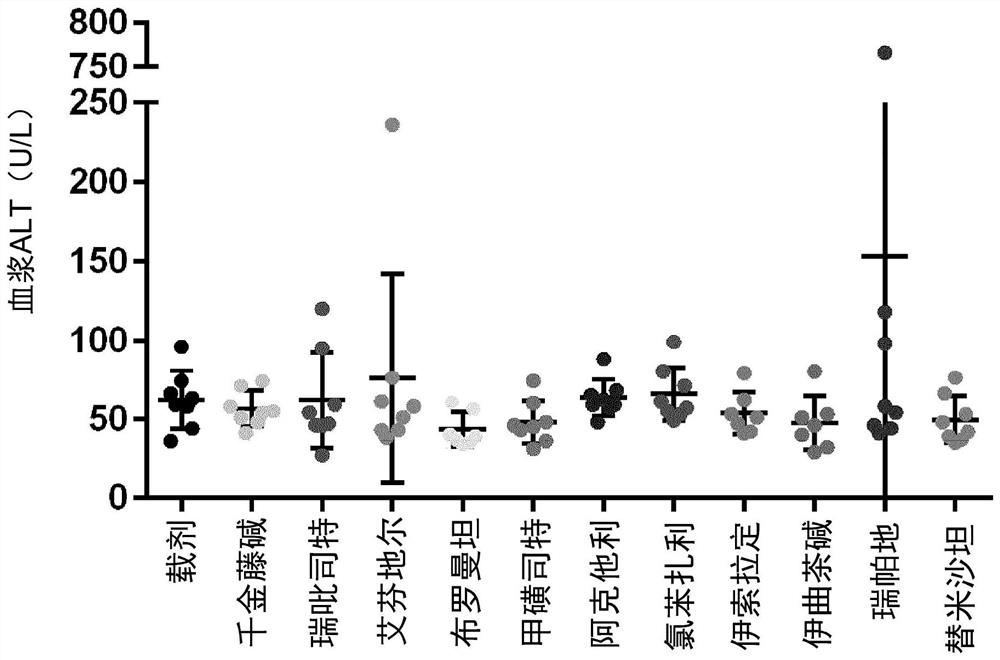
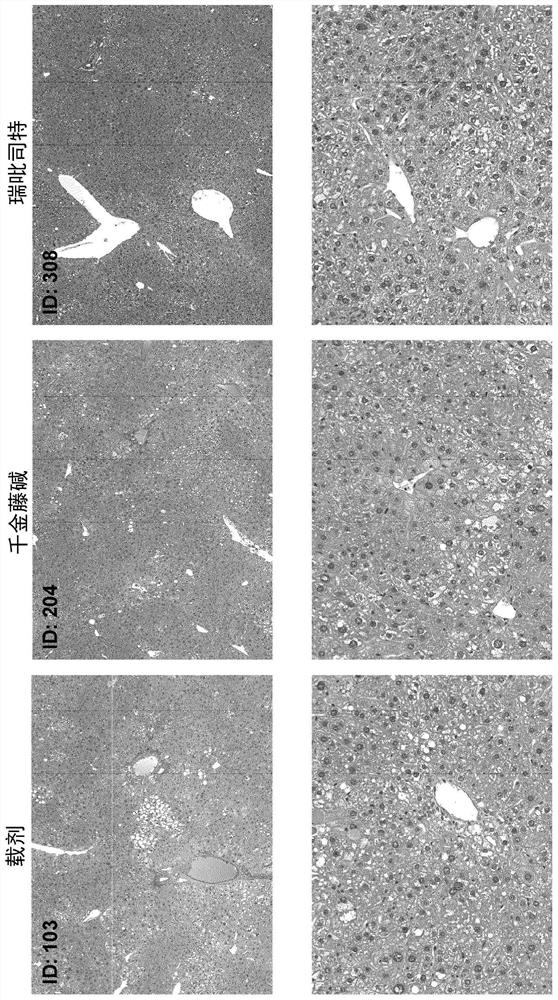
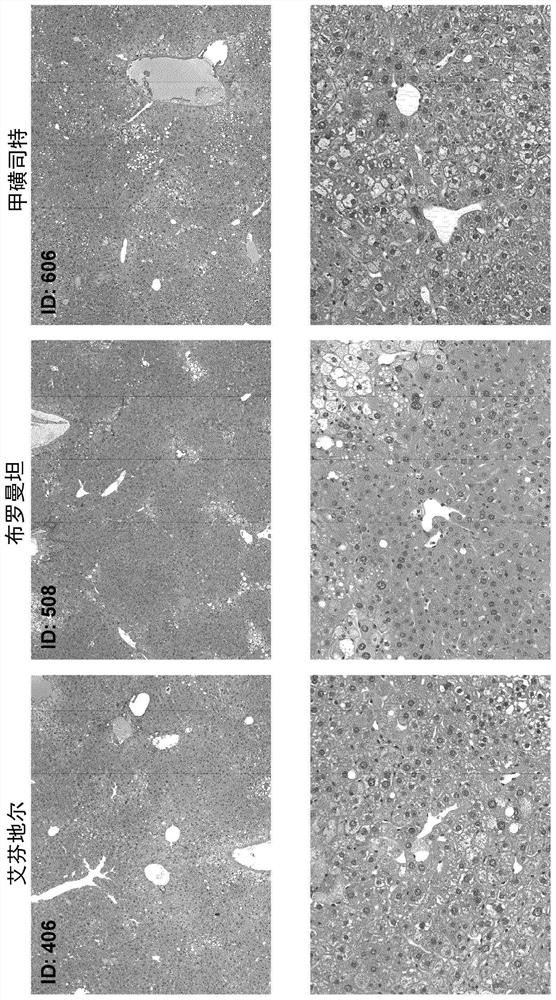
Examples
Embodiment 1
[0125] Materials and methods
[0126] Male neonatal C57BL / 6 mice were used. All mice were obtained from pathogen-free 14-day pregnant mice obtained from Japan SLC, Inc. (Hamamatsu, Japan) before the study began.
[0127] Murine STAM for NASH-HCC was performed according to previously described methods known in the art TM Model (Takakura et al., Characterization of non-alcoholic steatohepatitis, Anticancer Res, 34(9):4849-55 (2014); Fujii et al. (2013)). Male mice were given a single subcutaneous injection of 200 μg streptozotocin (STZ, Sigma, Missouri, USA) at 2 days after birth and fed a high-fat diet (CLEA Japan Inc) ad libitum with continuous feeding. , Tokyo, Japan) after 4 weeks of age (day 28±2), male mice were induced with NASH.
[0128] After induction of NASH, mice were randomly divided into 12 separate study groups of 8 6-week-old (42±2 days) mice based on body weight on the day before treatment initiation.
[0129] One day after randomization, mice were given o...
Embodiment 2
[0156] Materials and methods
[0157] Male neonatal C57BL / 6 mice were used as previously described. All mice were obtained from pathogen-free 14-day pregnant mice obtained from Japan SLC, Inc. (Hamamatsu, Japan) before the study began.
[0158] Murine STAM for NASH-HCC was performed according to previously described methods known in the art TM Model (Takakura et al., Characterization of non-alcoholic steatohepatitis, Anticancer Res, 34(9):4849-55 (2014); Fujii et al. (2013)) . Male mice were given a single subcutaneous injection of 200 μg streptozotocin (STZ, Sigma, Missouri, USA) at 2 days after birth and fed a high-fat diet (CLEA Japan Inc) ad libitum with continuous feeding. , Tokyo, Japan) after 4 weeks of age (day 28±2), male mice were induced with NASH.
[0159] After induction of NASH, the mice were randomly divided into 7 separate study groups of 8 6-week-old (42 ± 2 days) mice according to the body weight of the mice on the day before the start of treatment.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com