Methods of treating or preventing amyotrophic lateral sclerosis
A therapeutic agent, subject technology, applied in the field of treatment or prevention of amyotrophic lateral sclerosis, which can solve the problem of undetermined long-term efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0089] Pharmaceutical compositions useful in the methods of the invention may be suitably developed for inhalation, oral, rectal, vaginal, parenteral, topical, transdermal, pulmonary, intranasal, buccal, ophthalmic, intrathecal, intracranial, Intravenously or another route of administration. Other contemplated formulations include projected nanoparticles, liposomal formulations, resealed red blood cells containing active ingredients, and immunology-based formulations. The route or routes of administration will be apparent to those skilled in the art and will depend on any number of factors, including the type and severity of the disease being treated, the type and age of the veterinary patient or human patient being treated Wait. The formulation of the pharmaceutical compositions described herein can be prepared by any method known or hereafter developed in the art of pharmacology. In general, such preparation methods comprise the steps of bringing into association the activ...
example 1
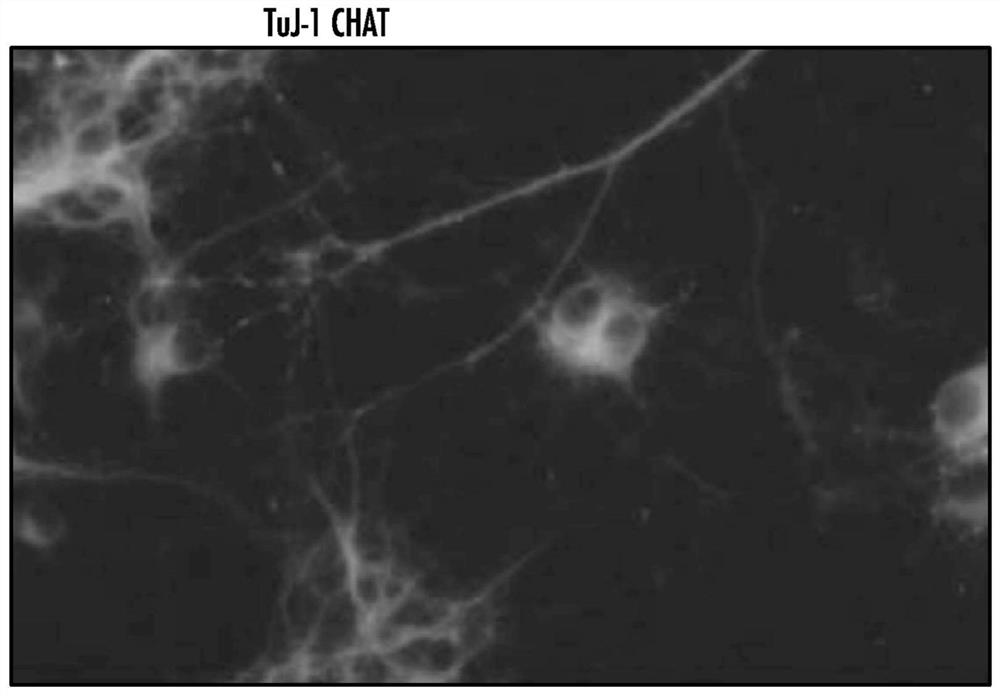
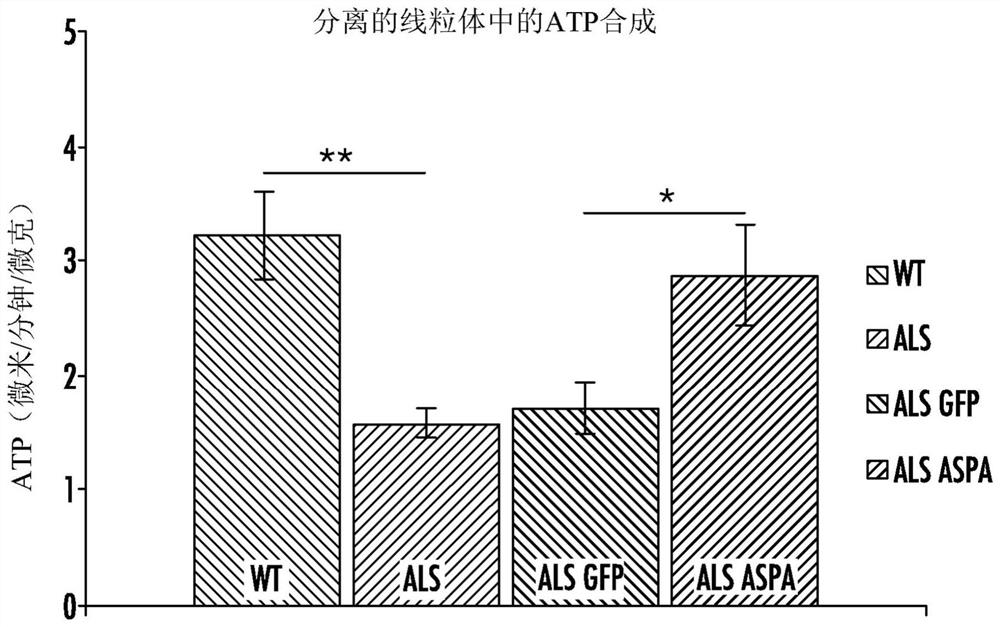
[0206] All starting materials were obtained from commercial suppliers and used without purification unless otherwise stated. Patient-derived induced pluripotent stem cells (iPSCs) were obtained from an NIH-funded biorepository (www.nimhgenetics org / available_data / ipsc / ) as described herein. Both normal healthy cells and cells from individuals carrying a mutation in SOD1(N139K) and diagnosed with familial ALS were used. It has been established that differentiation protocols result in the culture of motor neurons after 40 days of treatment with a defined regimen of growth factors and specialized media ( figure 1 ). Therapeutic (ASPA) and control (GFP) genes were encapsulated into AAV vectors and cells from the initial cohort were treated to assess effects on mitochondrial function. Mitochondria isolated from SOD1 mutant motoneurons were shown to have lower rates of ATP synthesis compared to wild-type cells, and transduction of these mutant cells with AAV-ASPA resulted in sign...
example 2
[0208] Effects of ASPA-derived free on ATP synthesis in isolated mitochondria from the spinal cord of 16-week-old SOD G93A mice Analysis of Aspartic Acid Boost.
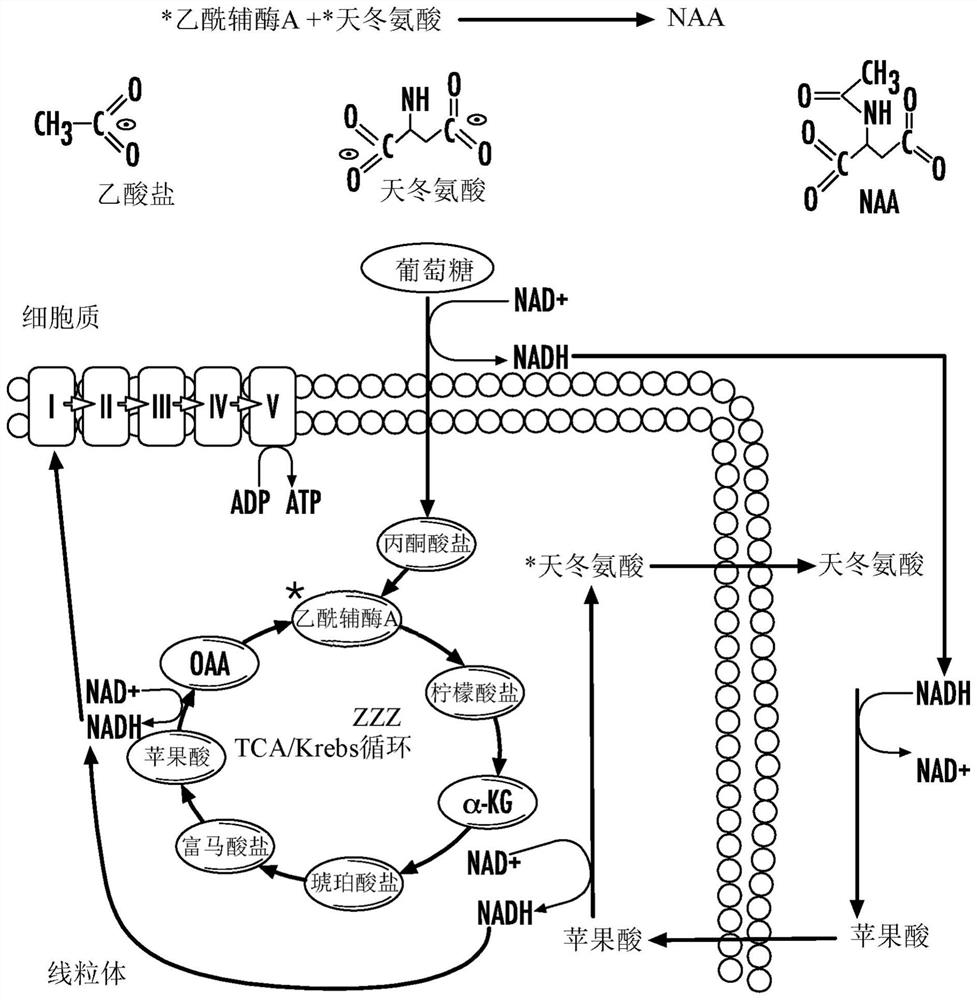
[0209] Mitochondria were isolated from whole 16-week-old SOD G93A spinal cords using mechanical homogenization and differential centrifugation. Mitochondria were kept on ice until used in the assay. The rate of ATP synthesis in 30 μg of isolated mitochondria was analyzed using a commercially available luminescence-based kit. A reaction mixture containing 1.0 mM malic acid, 1.0 mM glutamate, 10 mM NADH, and 0.2 mM ADP was prepared in a solution containing luciferase and luciferin, and 30 μg of mitochondria was added. Addition of 2 U / ml aspartate aminotransferase and 3 U / ml malate dehydrogenase drives MAS and initiates ATP synthesis. In this example, follow the Figure 8 Protocol in , free aspartic acid was substituted for the reaction product generated by incubation of lysates from cells overexpressing wild-typ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap