A method for synthesizing nucleoside analogs in a continuous flow reactor
A technology of nucleoside analogues and reactors, which is applied in the direction of organic chemistry, can solve problems such as difficult recrystallization and purification processes, complicated temperature control operations, and potential safety hazards, so as to reduce incomplete deprotection of hydroxyl groups and side reactions, and improve reaction efficiency. Efficiency and safety improvement effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
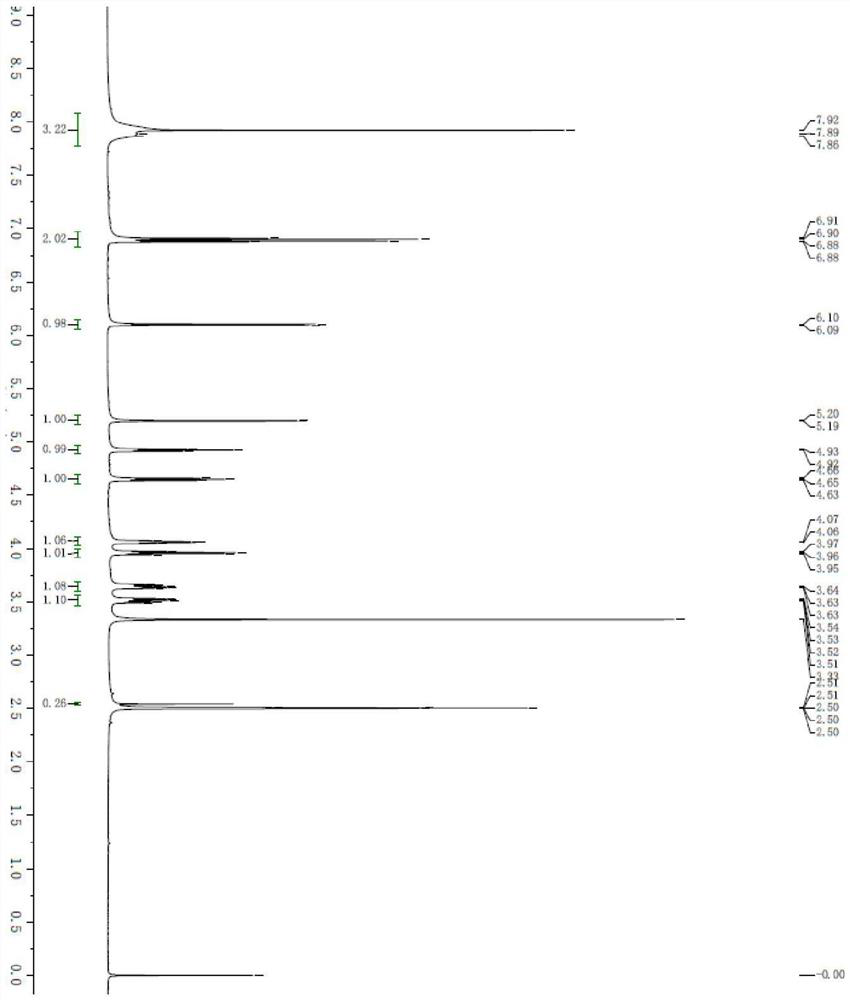
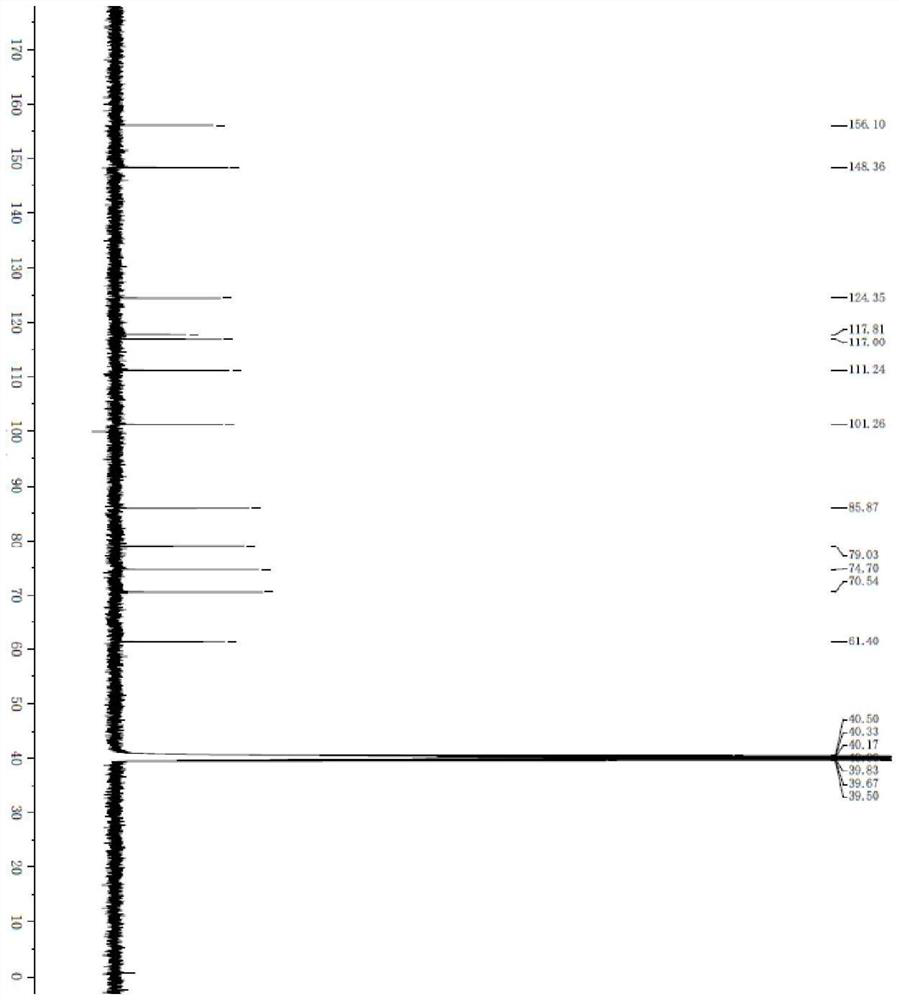
[0041] A continuous flow reactor for the synthesis of (2R, 3R, 4S, 5R)-2-(4-aminopyrrole[1,2-f][1,2,4]triazin-7-yl)-3,4 -The method of dihydroxyl-5-(hydroxymethyl)tetrahydrofuran-2-carbonitrile, the synthetic route is:
[0042]
[0043] Include the following steps:
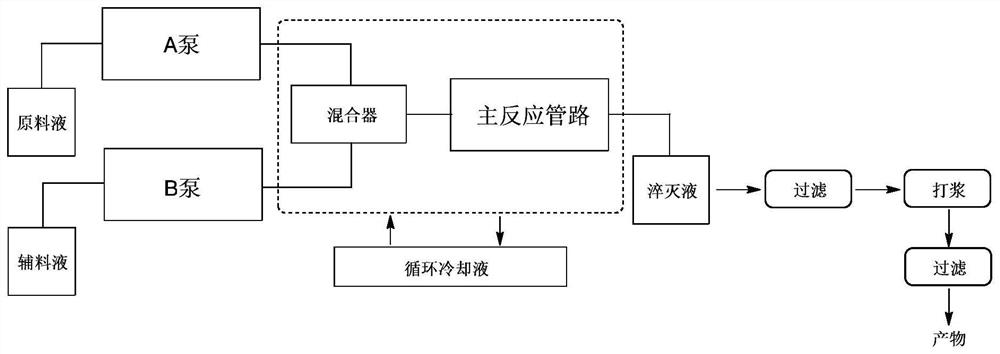
[0044] (1) Material preparation: Dissolve about 200g of the raw material in dichloromethane to prepare a 0.5mmol / mL raw material solution, and place it in a raw material solution bottle; 1.0mmol / mL boron trichloride dichloromethane solution (about 1200mL) Directly used as the auxiliary material liquid, placed in the auxiliary material liquid bottle; connect the raw material liquid bottle to the metering pump A; connect the auxiliary material liquid bottle to the metering pump B;
[0045] (2) Reaction temperature parameter setting: start the circulating liquid cooling system, so that the mixer and the main reaction pipeline are at a set temperature of about 5 degrees;
[0046](3) Material flow rate parameter s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com