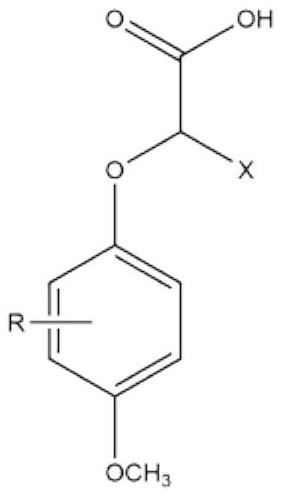
2-(4-methoxyphenoxy)propionic acid derivatives with sweet taste inhibitory effect and industrial production method thereof
A technology of methoxyphenoxy and propionic acid derivatives, which is applied in the field of food science, can solve the problems of high yield, difficult reaction raw materials, and low purity of reaction products, and achieve the effect of simple separation and purification steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of 2-(3,4-dimethoxyphenoxy)propionic acid
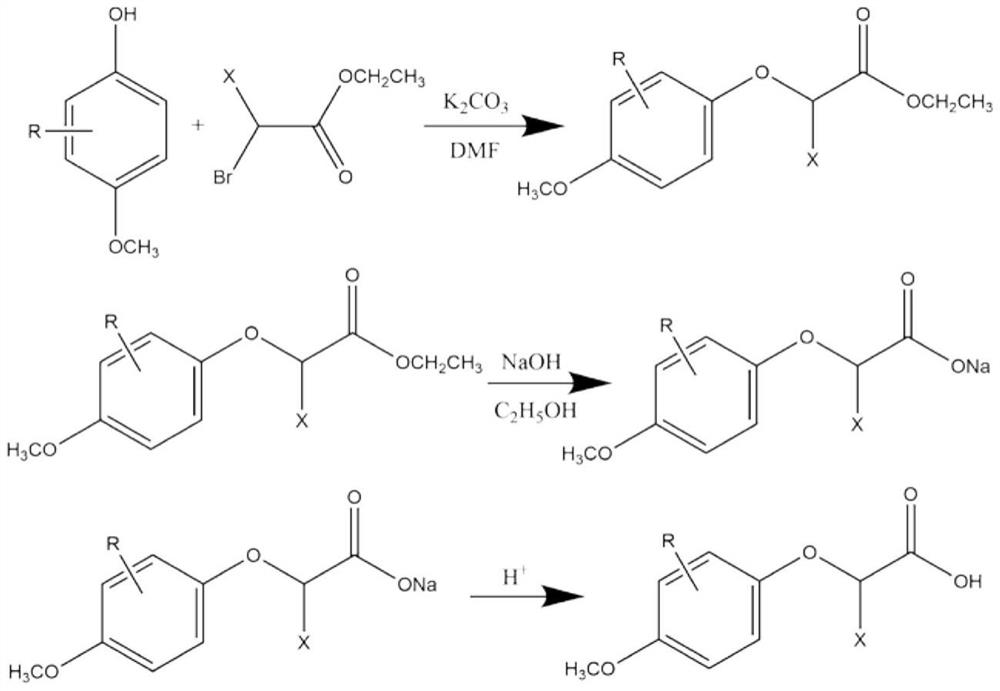
[0034] (1) Add 10kg of 3,4-dimethoxyphenol, 14kg of ethyl 2-bromopropionate, 13kg of potassium carbonate and 150L of dimethylformamide into the reaction vessel, and stir at 70°C for 11h. Cool and filter after the reaction. 400L of water and 500L of n-heptane were added to the filtrate, the layers were left to stand, and the collected organic layer was distilled to obtain ethyl 2-(3,4-dimethoxyphenoxy)propionate;
[0035] (2) The obtained ethyl 2-(3,4-dimethoxyphenoxy)propionate and 3kg of sodium hydroxide were added to 90kg of ethanol, and after reflux reaction at 85°C for 3h, the solvent was distilled off. The obtained solid was dissolved in water, and the pH was adjusted to 2 by adding hydrochloric acid solution with a mass concentration of 30%. The acidified mixture was extracted with ether, and saturated Na 2 CO 3 The collected organic layers were extracted. The pH of the finally collected water layer was ...
Embodiment 2
[0039] Preparation of 2-(2-chloro-4-methoxyphenoxy)propionic acid
[0040] (1) Add 10kg of 2-chloro-4-methoxyphenol, 14kg of ethyl 2-bromopropionate, 13kg of potassium carbonate and 150L of dimethylformamide into a reaction vessel, and stir at 70°C for 11 hours. Cool and filter after the reaction. 400L of water and 500L of n-heptane were added to the filtrate, the layers were left to stand, and the organic layer collected by distillation was obtained to obtain ethyl 2-(2-chloro-4-methoxyphenoxy)propionate;
[0041] (2) The obtained ethyl 2-(2-chloro-4-methoxyphenoxy)propionate and 3kg of sodium hydroxide were added to 90kg of ethanol, and after reflux at 85°C for 3h, the solvent was distilled off. The obtained solid was dissolved in water, and the pH was adjusted to 2 by adding hydrochloric acid solution with a mass concentration of 30%. The acidified mixture was extracted with ether, and saturated Na 2 CO 3 The collected organic layers were extracted. The final collected...
Embodiment 3
[0045] 2-(4-Methoxy-2-methylphenoxy)acetic acid
[0046] (1) Add 10kg of 2-methyl-4-methoxyphenol, 14kg of ethyl 2-bromoacetate, 13kg of potassium carbonate and 150L of dimethylformamide into a reaction vessel, and stir at 70°C for 11 hours. Cool and filter after the reaction. 400L of water and 500L of n-heptane were added to the filtrate, the layers were left to stand, and the collected organic layer was distilled to obtain ethyl 2-(4-methoxy-2-methylphenoxy)acetate;
[0047] (2) The obtained ethyl 2-(4-methoxy-2-methylphenoxy)acetate and 3kg of sodium hydroxide were added to 90kg of ethanol, and after reflux at 85°C for 3h, the solvent was distilled off. The obtained solid was dissolved in water, and the pH was adjusted to 2 by adding hydrochloric acid solution with a mass concentration of 30%. The acidified mixture was extracted with ether, and saturated Na 2 CO 3 The collected organic layers were extracted. Adjust the pH of the finally collected water layer to 2 with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com