Composition for preventing and/or treating influenza
A composition, influenza technology, application in medicine, composition of cannabinoid compounds, and the field of compositions for preventing and treating influenza, can solve problems such as poor, patients have no therapeutic effect, etc., and achieve suppression of influenza Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Embodiment 1 full-spectrum oil preparation process
[0081] (1) Take industrial hemp flowers and leaves and grind them, pass through No. 1 sieve, bake at 100°C for 200 minutes, take a certain amount of roasted hemp flowers and leaves, and add 70% of them according to the material-to-liquid ratio of 1:8 (w / v) Ethanol, stirring and extracting twice at room temperature, each time for 1 hour, filtering, combining the extracts, centrifuging, and concentrating the centrifuged filtrate under reduced pressure (65°C, -0.08~-0.09Mpa) until there is no alcohol smell and the density is 1.070 (60°C ), add purified water until the weight of the solution is 8 times the weight of the medicinal material, stir evenly, and then the sample solution is obtained.
[0082] (2) Measure the sample solution, purify it through the treated macroporous resin column (diameter-to-height ratio: 1:5) at a flow rate of 4BV / h, let it stand for 60 minutes after loading, and then use a flow rate of 9BV / h i...
Embodiment 2
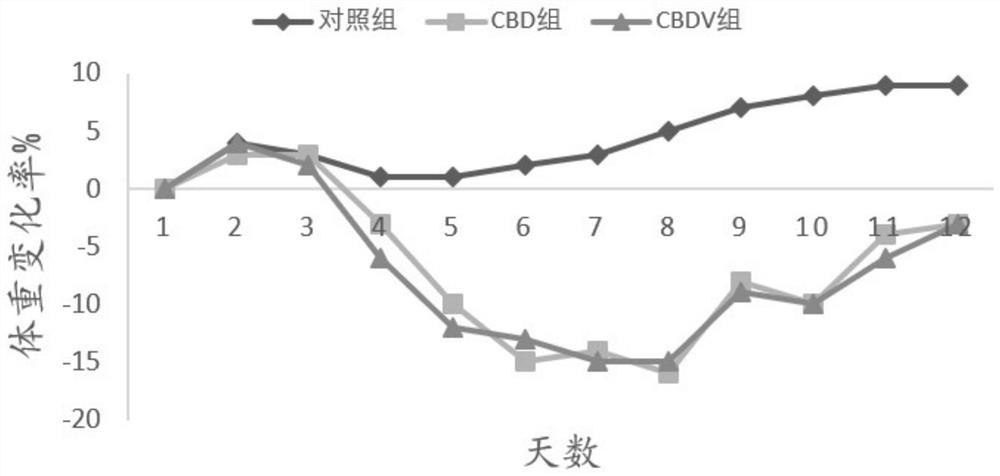
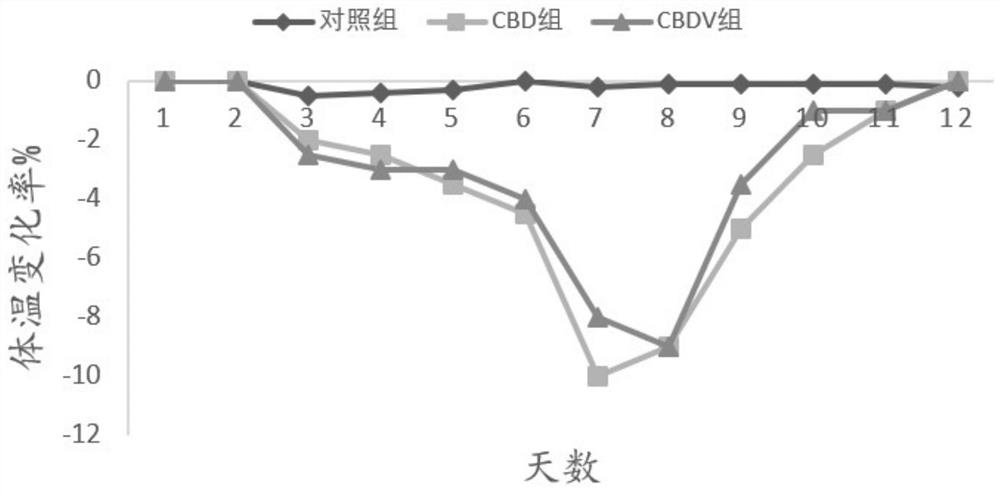
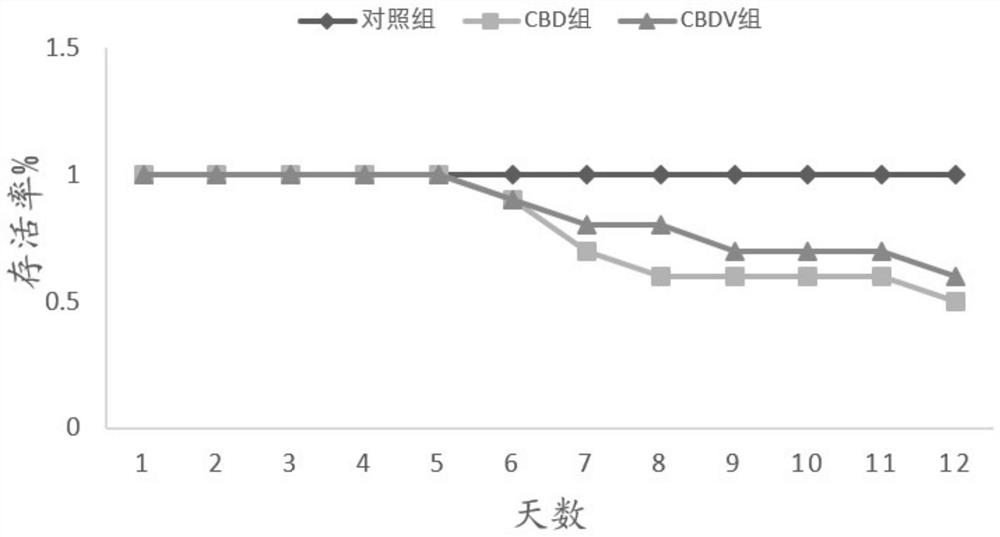
[0085] Application of embodiment 2CBDV in anti-influenza virus
[0086] 1. Amplification of H1N1 WSN influenza virus
[0087] (1) Select 9-day-old SPF chicken embryos, draw the position of the air chamber, find the position with fewer blood vessels and draw the line;
[0088] (2) Disinfect the shell of the chicken embryo with iodophor and 90% ethanol, punch a hole at 2 mm above the scribed position in step (1), get 150 μl of H1N1 WSN influenza virus diluted in PBS and inject it into the allantoic cavity of the chicken embryo, and wax it The punched hole is sealed;
[0089] (3) Place the chicken embryos in step (2) at 37°C for 48h-72h and observe the survival of the chicken embryos every 24h. If the death of the chicken embryos is observed, put the chicken embryos at 4°C overnight and collect the allantoic fluid. At 72 hours, place the remaining chicken embryos at 4°C overnight and collect the allantoic fluid;
[0090] (4) The allantoic fluid harvested in step (3) was centri...
Embodiment 3
[0109] The application of the composition of embodiment 3 cannabinoid compound in anti-influenza virus
[0110] 1. Animals are infected with H1N1 WSN virus and administered
[0111] Amplify H1N1 WSN virus and titer determination according to the mode of embodiment 1, and select Yunnan white male mice according to the mode of embodiment 1, be divided into 11 groups at random, each group 10, concrete grouping is as follows:
[0112] CBDV+CBD combination group: Intranasal administration of H1N1 WSN influenza virus 50μl / head, and intraperitoneal injection on the fourth day, CBDV 2.5mg / kg+CBD 20mg / kg, CBDV 3mg / kg+CBD20mg / kg, CBDV 3.5mg / kg+CBD20mg / kg;
[0113] CBDV+CBD+CBG combination group: Intranasal administration of H1N1 WSN influenza virus 50μl / head, and intraperitoneal injection on the fourth day, CBDV 3mg / kg+CBD 20mg / kg+CBG 0.05mg / kg, CBDV 3mg / kg+ CBD 20mg / kg+CBG0.25mg / kg, CBDV 3mg / kg+CBD 20mg / kg+CBG0.5mg / kg;
[0114] CBDV+CBD+THCV combination group: Intranasal administra...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap