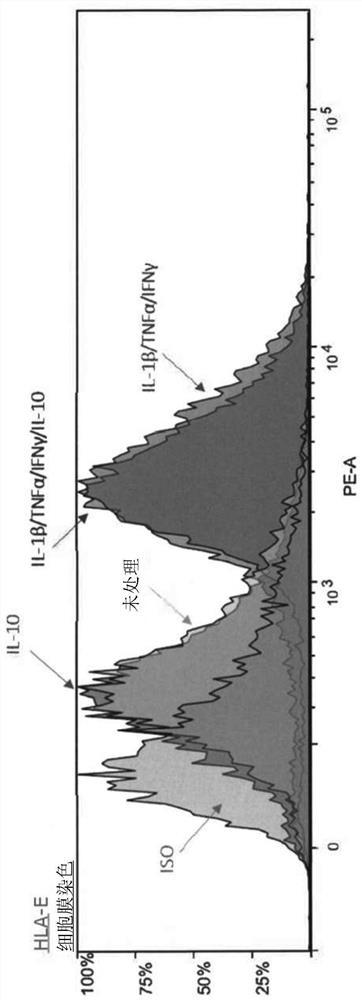
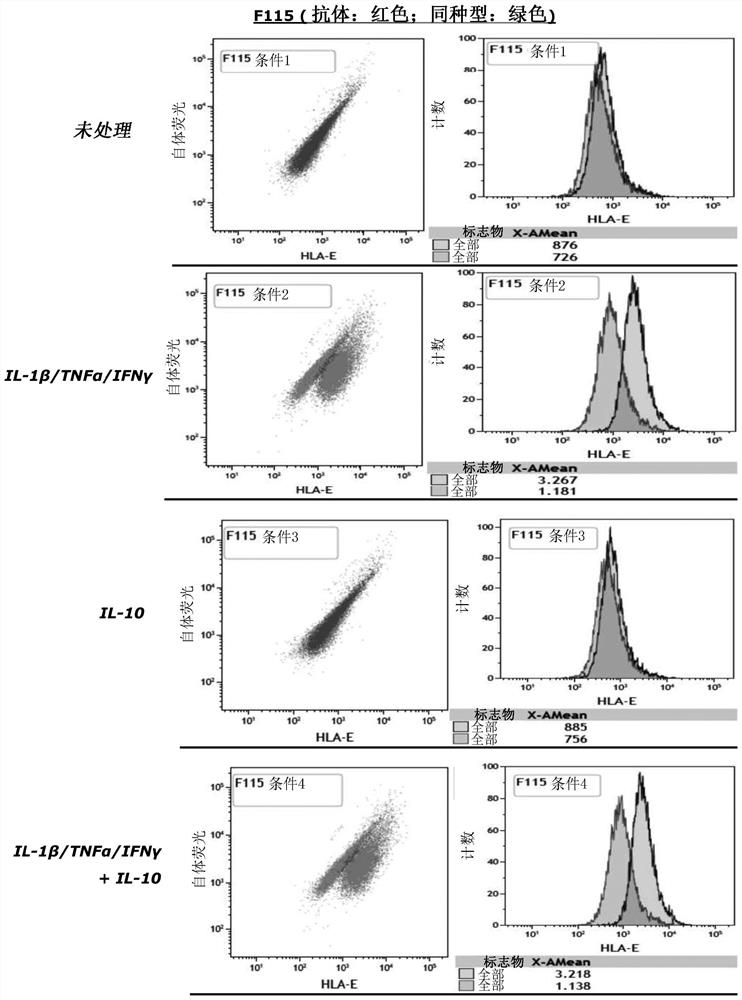
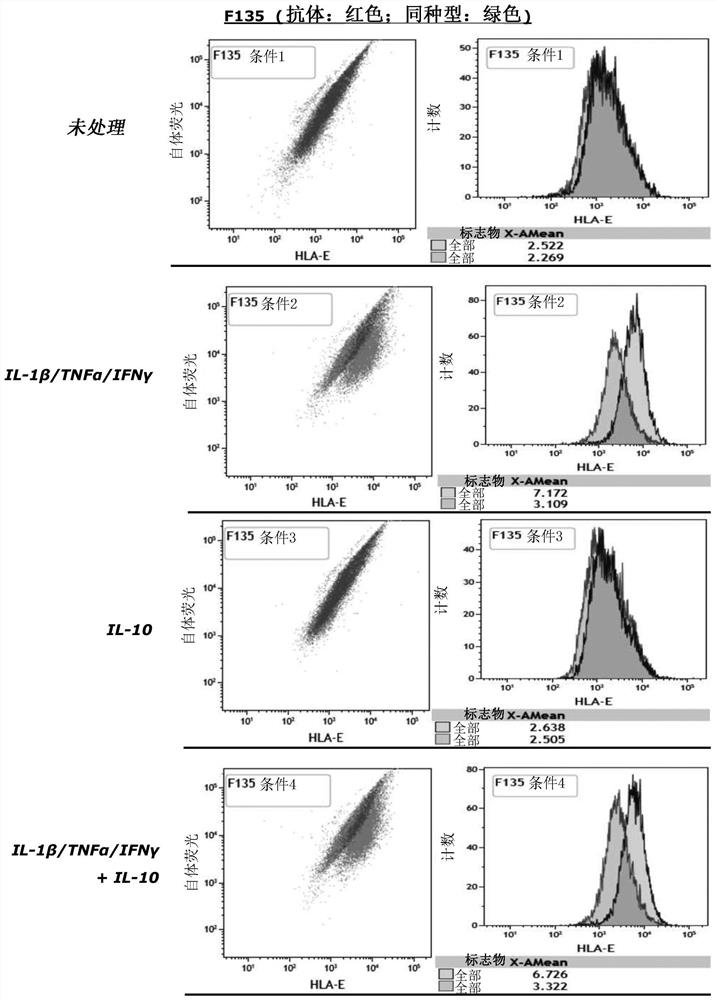
Cell composition comprising liver progenitor cells expressing hla-e
A technology of HLA-E and composition, applied in the direction of liver cells, drug combinations, animal cells, etc., can solve the problems of not disclosing liver stem cells or progenitor cell populations, not mentioning HLA-E expression, etc., to increase efficacy and safety Sexual, potentiated immunosuppressive and/or immune-evading properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0207] 1. Preparation of pretreated HLA-E positive hepatic progenitor cells or stem cells
[0208] (a) Preparation of hepatic progenitor cells
[0209] Human adult hepatic progenitor cells were isolated from the liver of healthy cadaver or beating donors as described in EP3140393 or WO2017149059.
[0210] Briefly, hepatocyte preparations were resuspended in Williams'E medium supplemented with 10% FBS, 10 mg / ml INS, 1 mM DEX. primary cells in Flasks were cultured at 37°C in 5% CO 2 cultured in a completely humid atmosphere. After 24 hours, the medium was changed to eliminate non-adherent cells, and refreshed twice a week thereafter, and the cultures were observed daily under the microscope. After 12-16 days, change the medium to high glucose DMEM supplemented with 9% FBS. Cell types with mesenchymal-like morphology emerge and proliferate. When reaching 70-95% confluence, cells were trypsinized with recombinant trypsin and 1 mM EDTA and incubated at 1-10 x 10 3 cells / c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com