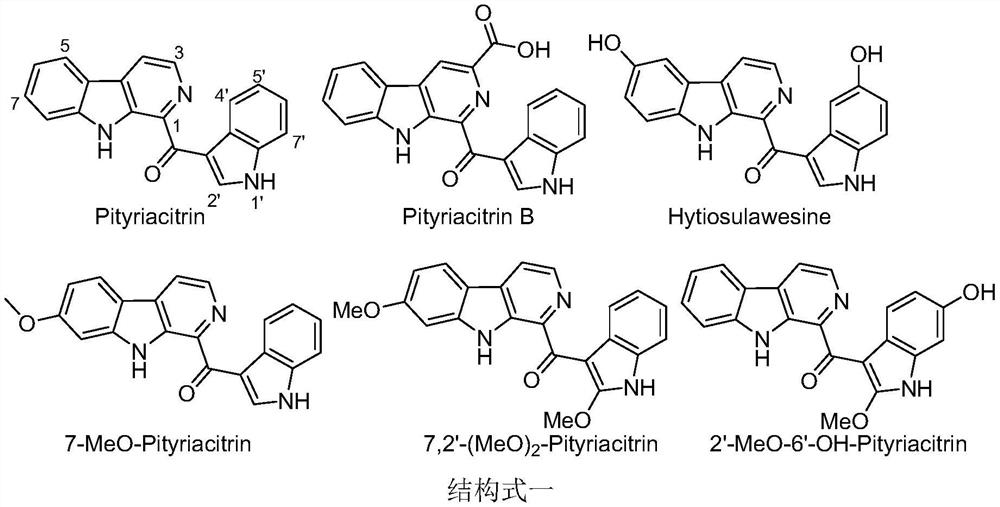
Pityriacitrin alkaloid derivative containing acylhydrazone structure and its preparation method and use
A technology of alkaloid derivatives and acylhydrazones, which is applied in the field of pityriacitrin alkaloid derivatives containing an acylhydrazone structure and its preparation, can solve the problems of high cost, low natural content of pityriacitrins alkaloids, low synthesis yield, etc., and achieve good results Anti-Plant Virus and Pathogen Activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
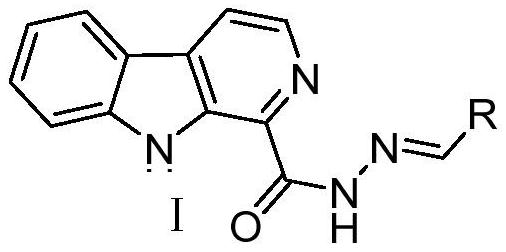
[0026] The preparation method of the pityriacitrin alkaloid derivative containing the acylhydrazone structure with the chemical structural formula I is as follows:
[0027] The prepared pityriacitrin alkaloid derivatives containing an acylhydrazone structure have the following chemical structure formula I:
[0028]
[0029] In the above chemical structural formula I, R represents methyl, propyl, isopropyl, n-butyl, n-butyl, tert-butyl, benzyl, cyclopentyl, cyclohexyl, phenyl, 2-fluorophenyl , 3-fluorophenyl, 3,4-difluorophenyl, 2,6-difluorophenyl, 2,4-difluorophenyl, 2,3-difluorophenyl, 4-fluorophenyl, 2 -Chlorophenyl, 3-chlorophenyl, 3,4-dichlorophenyl, 2,6-dichlorophenyl, 2,4-dichlorophenyl, 2,3-dichlorophenyl, 2,5 -Dichlorophenyl, 4-chlorophenyl, 5-chloro-2-fluorophenyl, 2-bromophenyl, 3-bromophenyl, 4-bromophenyl, 4-iodophenyl, 2-nitro Phenyl, 3-nitrophenyl, 4-nitrophenyl, 2-methylphenyl, 3-methylphenyl, 4-methylphenyl, 2-trifluoromethylphenyl, 3-tri Fluoromethylphen...
Embodiment 2
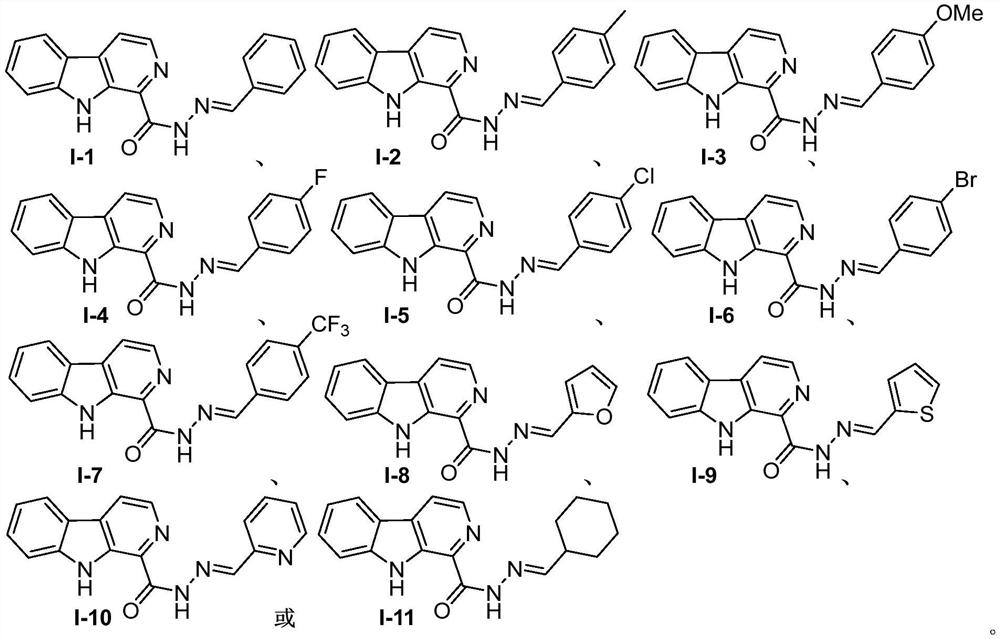
[0034] The preparation method of N'-benzylidene-pyrido[3,4-b]indole-1-carboxylhydrazide shown in chemical structural formula I-1 is as follows:
[0035] The chemical structural formula I-1 of N'-benzylidene-pyrido[3,4-b]indole-1-formyl hydrazide is
[0036]
[0037] The concrete steps of its preparation method are as follows:
[0038] The first step, after mixing the aqueous solution of glyoxylic acid monohydrate (10.37g, mass fraction 50%) and ethyl acetate (145mL), add K 2 CO 3 An aqueous solution (20 mL, 10% by mass) was adjusted to pH 5, and tryptamine (9.60 g, 60 mmol) was slowly added under stirring to fully dissolve it, and then stirred and reacted at room temperature for 24 hours. After the reaction, filter, and wash the solid product with ethyl acetate and a small amount of ethanol, and dry to obtain 10.702g of yellow solid compound 1, with a yield of 83%. After determination, the relevant parameters of the yellow solid are: 1 H NMR (400MHz, DMSO-d 6 )δ2.81–2.8...
Embodiment 3
[0044] The preparation method of N'-(4-methylbenzylidene)-pyrido[3,4-b]indole-1-carboxylhydrazide shown in chemical structural formula I-2 is as follows:
[0045] The chemical structural formula I-2 of N'-(4-methylbenzylidene)-pyrido[3,4-b]indole-1-formyl hydrazide is
[0046]
[0047] The concrete steps of its preparation method are as follows:
[0048] The first step to the fourth step, with embodiment 1;
[0049] In the fifth step, except that 4-methylbenzaldehyde is used, the others are the same as in Example 1, and diethyl ether is recrystallized and purified to obtain a light yellow solid with a yield of 92%; after determination, the relevant parameters of the light yellow solid are: 1 H NMR (400MHz, DMSO-d 6 )δ11.24 (s,1H,CONH),11.83(s,1H,NH),8.69(s,1H,N=CH),8.44(d,J=4.3and 22.4Hz,2H,ArH),7.84( d,J=8.1Hz,1H,ArH),7.84(d,J=8.1Hz,1H,ArH),7.65(d,J=7.5Hz,2H,ArH),7.60(t,J=7.7Hz,1H ,ArH),7.30–7.28(m,2H,ArH),2.35(s,3H,CH 3 ); 13 C NMR (100MHz, DMSO-d 6 )δ161.7, 148.7,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com